Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets
- PMID: 34887515
- PMCID: PMC8656144
- DOI: 10.1038/s41556-021-00805-8
Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets
Abstract
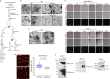
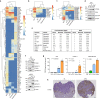
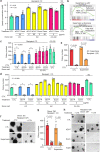
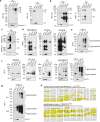
Extracellular vesicles and exomere nanoparticles are under intense investigation as sources of clinically relevant cargo. Here we report the discovery of a distinct extracellular nanoparticle, termed supermere. Supermeres are morphologically distinct from exomeres and display a markedly greater uptake in vivo compared with small extracellular vesicles and exomeres. The protein and RNA composition of supermeres differs from small extracellular vesicles and exomeres. Supermeres are highly enriched with cargo involved in multiple cancers (glycolytic enzymes, TGFBI, miR-1246, MET, GPC1 and AGO2), Alzheimer's disease (APP) and cardiovascular disease (ACE2, ACE and PCSK9). The majority of extracellular RNA is associated with supermeres rather than small extracellular vesicles and exomeres. Cancer-derived supermeres increase lactate secretion, transfer cetuximab resistance and decrease hepatic lipids and glycogen in vivo. This study identifies a distinct functional nanoparticle replete with potential circulating biomarkers and therapeutic targets for a host of human diseases.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
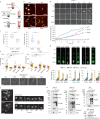



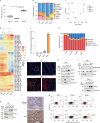
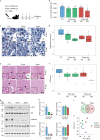
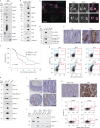



Comment in
-
Profiling and promise of supermeres.Nat Cell Biol. 2021 Dec;23(12):1217-1219. doi: 10.1038/s41556-021-00808-5. Nat Cell Biol. 2021. PMID: 34887514 Free PMC article.
References
-
- Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019;21:9–17. - PubMed
-
- van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

