Inhibition of Amyloid Aggregation and Toxicity with Janus Iron Oxide Nanoparticles
- PMID: 34887621
- PMCID: PMC8651233
- DOI: 10.1021/acs.chemmater.1c01947
Inhibition of Amyloid Aggregation and Toxicity with Janus Iron Oxide Nanoparticles
Abstract
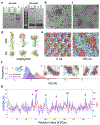
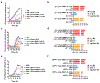
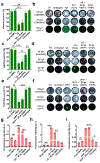
Amyloid aggregation is a ubiquitous form of protein misfolding underlying the pathologies of Alzheimer's disease (AD), Parkinson's disease (PD) and type 2 diabetes (T2D), three primary forms of human amyloid diseases. While much has been learned about the origin, diagnosis and management of these neurological and metabolic disorders, no cure is currently available due in part to the dynamic and heterogeneous nature of the toxic oligomers induced by amyloid aggregation. Here we synthesized beta casein-coated iron oxide nanoparticles (βCas IONPs) via a BPA-P(OEGA-b-DBM) block copolymer linker. Using a thioflavin T kinetic assay, transmission electron microscopy, Fourier transform infrared spectroscopy, discrete molecular dynamics simulations and cell viability assays, we examined the Janus characteristics and the inhibition potential of βCas IONPs against the aggregation of amyloid beta (Aβ), alpha synuclein (αS) and human islet amyloid polypeptide (IAPP) which are implicated in the pathologies of AD, PD and T2D. Incubation of zebrafish embryos with the amyloid proteins largely inhibited hatching and elicited reactive oxygen species, which were effectively rescued by the inhibitor. Furthermore, Aβ-induced damage to mouse brain was mitigated in vivo with the inhibitor. This study revealed the potential of Janus nanoparticles as a new nanomedicine against a diverse range of amyloid diseases.
Keywords: Amyloid beta; IAPP; Janus nanoparticle; alpha synuclein; iron oxide nanoparticle.
Conflict of interest statement
Competing financial interests The authors declare no conflicting financial interests.
Figures





References
-
- Hartl FU; Hayer-Hartl M, Converging concepts of protein folding in vitro and in vivo. Nat. Struct. Mol. Biol 2009, 16, 574–581. - PubMed
-
- Clark A; Lewis CE; Willis AC; Cooper GJS; Morris JF; Reid KBM; Turner RC, Islet amyloid formed from diabetes-associated peptide may be pathogenic in type-2 diabetes. The Lancet 1987, 330, 231–234. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
