Clinical characteristics of gastrointestinal immune-related adverse events of immune checkpoint inhibitors and their association with survival
- PMID: 34887637
- PMCID: PMC8613649
- DOI: 10.3748/wjg.v27.i41.7190
Clinical characteristics of gastrointestinal immune-related adverse events of immune checkpoint inhibitors and their association with survival
Abstract
Background: Despite the popularity of immune checkpoint inhibitors (ICIs) in the treatment of advanced cancer, patients often develop gastrointestinal (GI) and non-GI immune-related adverse events (irAEs). The clinical characteristics and survival outcomes of GI-irAEs have not been fully elucidated in previous reports. This necessitates the evaluation of the impact of GI-irAEs on patients receiving ICI treatment.
Aim: To evaluate the clinical characteristics of GI-irAEs and their impact on survival in patients treated with ICIs.
Methods: In this single-center, retrospective, observational study, we reviewed the records of 661 patients who received ICIs for various cancers at Nagoya University Hospital from September 2014 to August 2020. We analyzed the clinical characteristics of patients who received ICI treatment. We also evaluated the correlation between GI-irAE development and prognosis in non-small cell lung cancer (LC) and malignant melanoma (MM). Kaplan-Meier analysis was used to compare the median overall survival (OS). Multivariate Cox proportional hazards models were used to identify prognostic factors. A P value < 0.05 was considered statistically significant.
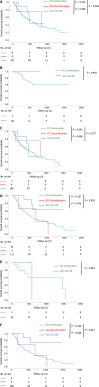
Results: GI-irAEs occurred in 34 of 605 patients (5.6%) treated with an anti-programmed cell death-1/programmed death-ligand 1 (anti-PD-1/PD-L1) antibody alone and in nine of 56 patients (16.1%) treated with an anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) antibody alone or a combination of anti-PD-1 and anti-CTLA-4 antibodies. The cumulative incidence and median daily diarrhea frequency were significantly higher in patients receiving anti-CTLA-4 antibodies (P < 0.05). In 130 patients with MM, OS was significantly prolonged in the group that continued ICI treatment despite the development of GI-irAEs compared to the group that did not experience GI-irAEs (P = 0.035). In contrast, in 209 patients with non-small cell LC, there was no significant difference in OS between the groups. The multivariate analyses showed that a performance status of 2-3 (hazard ratio: 2.406; 95% confidence interval: 1.125-5.147; P = 0.024) was an independent predictive factor for OS in patients with MM.
Conclusion: Patients receiving anti-CTLA-4 antibodies develop GI-irAEs more frequently and with higher severity than those receiving anti-PD-1/PD-L1 antibodies. Continuing ICI treatment in patients with MM with GI-irAEs have better OS.
Keywords: Colitis; Cytotoxic T-lymphocyte antigen 4; Diarrhea; Drug-related side effects and adverse reactions; Immune checkpoint inhibitors; Prognosis.
©The Author(s) 2021. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: Hase T received personal fees from AstraZeneca, Chugai Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd., and Bristol-Myers Squibb Co. and grants from AstraZeneca and Chugai Pharmaceutical Co. Ltd., outside the submitted work. Hashimoto N received a grant from Boehringer Ingelheim, outside the submitted work. Ando Y received grants from Mochida Pharmaceutical Co. Ltd.; grants and personal fees from Chugai Pharmaceutical Co. Ltd., Kyowa Kirin Co. Ltd., Nippon Kayaku Co. Ltd., Yakult Honsha Co. Ltd., Ono Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co. Ltd., Daiichi Sankyo Company Ltd., Eisai Co. Ltd.; and personal fees from Eli Lilly Japan K.K., Novartis Pharma K.K., Bayer Holding Ltd., Bristol-Myers Squibb, Sawai Pharmaceutical Co. Ltd., Tsumura & Co., Otsuka Holdings Co. Ltd., Roche Diagnostics K.K., AstraZeneca K.K., and MSD K.K, outside the submitted work. We declare that there are no conflict of interest.
Figures
Similar articles
-
Impact of immune-related adverse event severity on overall survival in patients with advanced NSCLC receiving immune checkpoint inhibitors therapy, with a focus on combination regimens.Lung Cancer. 2025 Jun;204:108555. doi: 10.1016/j.lungcan.2025.108555. Epub 2025 Apr 22. Lung Cancer. 2025. PMID: 40311310
-
Multisystem Immune-Related Adverse Events Associated With Immune Checkpoint Inhibitors for Treatment of Non-Small Cell Lung Cancer.JAMA Oncol. 2020 Dec 1;6(12):1952-1956. doi: 10.1001/jamaoncol.2020.5012. JAMA Oncol. 2020. PMID: 33119034 Free PMC article.
-
Prevalence of immune-related adverse events and anti-tumor efficacy following immune checkpoint inhibitor therapy in Japanese patients with various solid tumors.BMC Cancer. 2022 Nov 29;22(1):1232. doi: 10.1186/s12885-022-10327-7. BMC Cancer. 2022. PMID: 36447159 Free PMC article.
-
Risk of Ophthalmic Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis.Ocul Immunol Inflamm. 2022 Aug;30(6):1449-1459. doi: 10.1080/09273948.2021.1890133. Epub 2021 May 10. Ocul Immunol Inflamm. 2022. PMID: 33970759
-
Treatment- and immune-related adverse events of immune checkpoint inhibitors in advanced lung cancer.Biosci Rep. 2020 May 29;40(5):BSR20192347. doi: 10.1042/BSR20192347. Biosci Rep. 2020. PMID: 32315071 Free PMC article.
Cited by
-
Single-cell RNA sequencing analysis of peripheral blood mononuclear cells in PD-1-induced renal toxicity in patients with lung cancer.BMC Nephrol. 2024 Sep 14;25(1):307. doi: 10.1186/s12882-024-03754-0. BMC Nephrol. 2024. PMID: 39277735 Free PMC article.
-
Pharmacological Treatments Available for Immune-Checkpoint-Inhibitor-Induced Colitis.Biomedicines. 2022 Jun 6;10(6):1334. doi: 10.3390/biomedicines10061334. Biomedicines. 2022. PMID: 35740355 Free PMC article. Review.
-
Potential application mechanism of traditional Chinese medicine in treating immune checkpoint inhibitor-induced colitis.Front Immunol. 2024 Apr 10;15:1366489. doi: 10.3389/fimmu.2024.1366489. eCollection 2024. Front Immunol. 2024. PMID: 38660314 Free PMC article. Review.
-
Local Delivery of Immunomodulatory Antibodies for Gastrointestinal Tumors.Cancers (Basel). 2023 Apr 18;15(8):2352. doi: 10.3390/cancers15082352. Cancers (Basel). 2023. PMID: 37190279 Free PMC article. Review.
-
Endoscopic insights into digestive-related adverse effects of immune checkpoint inhibitors: A narrative review.World J Gastrointest Endosc. 2025 Jul 16;17(7):107798. doi: 10.4253/wjge.v17.i7.107798. World J Gastrointest Endosc. 2025. PMID: 40677571 Free PMC article. Review.
References
-
- Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. - PMC - PubMed
-
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbé C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. - PubMed
-
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hogg D, Hill A, Márquez-Rodas I, Haanen J, Guidoboni M, Maio M, Schöffski P, Carlino MS, Lebbé C, McArthur G, Ascierto PA, Daniels GA, Long GV, Bastholt L, Rizzo JI, Balogh A, Moshyk A, Hodi FS, Wolchok JD. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381:1535–1546. - PubMed
-
- Motzer RJ, Escudier B, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Plimack ER, Procopio G, McDermott DF, Castellano D, Choueiri TK, Donskov F, Gurney H, Oudard S, Richardet M, Peltola K, Alva AS, Carducci M, Wagstaff J, Chevreau C, Fukasawa S, Tomita Y, Gauler TC, Kollmannsberger CK, Schutz FA, Larkin J, Cella D, McHenry MB, Saggi SS, Tannir NM. Nivolumab vs everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer. 2020;126:4156–4167. - PMC - PubMed
-
- Geukes Foppen MH, Rozeman EA, van Wilpe S, Postma C, Snaebjornsson P, van Thienen JV, van Leerdam ME, van den Heuvel M, Blank CU, van Dieren J, Haanen JBAG. Immune checkpoint inhibition-related colitis: symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3:e000278. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

