Neural Infection by Oropouche Virus in Adult Human Brain Slices Induces an Inflammatory and Toxic Response
- PMID: 34887719
- PMCID: PMC8651276
- DOI: 10.3389/fnins.2021.674576
Neural Infection by Oropouche Virus in Adult Human Brain Slices Induces an Inflammatory and Toxic Response
Abstract
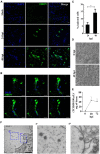
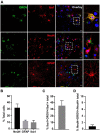
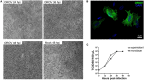
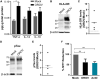
Oropouche virus (OROV) is an emerging arbovirus in South and Central Americas with high spreading potential. OROV infection has been associated with neurological complications and OROV genomic RNA has been detected in cerebrospinal fluid from patients, suggesting its neuroinvasive potential. Motivated by these findings, neurotropism and neuropathogenesis of OROV have been investigated in vivo in murine models, which do not fully recapitulate the complexity of the human brain. Here we have used slice cultures from adult human brains to investigate whether OROV is capable of infecting mature human neural cells in a context of preserved neural connections and brain cytoarchitecture. Our results demonstrate that human neural cells can be infected ex vivo by OROV and support the production of infectious viral particles. Moreover, OROV infection led to the release of the pro-inflammatory cytokine tumor necrosis factor-alpha (TNF-α) and diminished cell viability 48 h post-infection, indicating that OROV triggers an inflammatory response and tissue damage. Although OROV-positive neurons were observed, microglia were the most abundant central nervous system (CNS) cell type infected by OROV, suggesting that they play an important role in the response to CNS infection by OROV in the adult human brain. Importantly, we found no OROV-infected astrocytes. To the best of our knowledge, this is the first direct demonstration of OROV infection in human brain cells. Combined with previous data from murine models and case reports of OROV genome detection in cerebrospinal fluid from patients, our data shed light on OROV neuropathogenesis and help raising awareness about acute and possibly chronic consequences of OROV infection in the human brain.
Keywords: arboviruses; histocultures; human brain; neuroinfection; neuroinflammation; neurotropic virus; viral encephalitis.
Copyright © 2021 Almeida, Souza, Mendes, Pontelli, Pinheiro, Nogueira, Cardoso, Paiva, Ferrari, Veras, Cunha, Horta-Junior, Alberici, Cunha, Podolsky-Gondim, Neder, Arruda and Sebollela.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Amorim M. R., Pontelli M. C., de Souza G. F., Muraro S. P., Toledo-Teixeira D. A., Forato J., et al. (2020). Oropouche virus infects, persists and induces IFN response in human peripheral blood mononuclear cells as identified by RNA PrimeFlow. And qRT-PCR assays. Viruses 12:200785. 10.3390/v12070785 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

