Inflammation and Platelet Activation After COVID-19 Vaccines - Possible Mechanisms Behind Vaccine-Induced Immune Thrombocytopenia and Thrombosis
- PMID: 34887867
- PMCID: PMC8649717
- DOI: 10.3389/fimmu.2021.779453
Inflammation and Platelet Activation After COVID-19 Vaccines - Possible Mechanisms Behind Vaccine-Induced Immune Thrombocytopenia and Thrombosis
Abstract
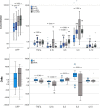
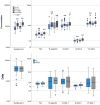
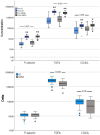
Introduction of vaccines against COVID-19 has provided the most promising chance to control the world-wide COVID-19 pandemic. However, the adenovirus-vector based Oxford/AstraZeneca [ChAdOx1] (AZ) and Johnson & Johnson [Ad26.CoV2.S] COVID-19 vaccines have been linked with serious thromboembolic events combined with thrombocytopenia, denominated Vaccine-induced Immune Thrombocytopenia and Thrombosis (VITT). The pathogenesis of COVID-19 VITT remain incompletely understood; especially the initial events that trigger platelet activation, platelet factor (PF)4 release, complex formation and PF4 antibody production are puzzling. This is a prospective study investigating the impact of different COVID-19 vaccines on inflammation (CRP, TNF-α, IL-1β, IL-6, IL-8, IL-10), vascular endothelial activation (syndecan-1, thrombomodulin, E-selectin, ICAM-1, ICAM-3, VCAM-1), platelet activation (P-selectin, TGF-β, sCD40L) and aggregation (Multiplate® impedance aggregometry), whole blood coagulation (ROTEM®), thrombin generation and PF4 antibodies to reveal potential differences between AZ and mRNA vaccines in individuals without VITT. The study included 80 (55 AZ and 55 mRNA) vaccinated individuals and 55 non-vaccinated age- and gender matched healthy controls. The main findings where that both vaccines enhanced inflammation and platelet activation, though AZ vaccination induced a more pronounced increase in several inflammatory and platelet activation markers compared to mRNA vaccination and that post-vaccination thrombin generation was higher following AZ vaccination compared to mRNA vaccination. No difference in neither the PF4 antibody level nor the proportion of individuals with positive PF4 antibodies were observed between the vaccine groups. This is the first study to report enhanced inflammation, platelet activation and thrombin generation following AZ vaccination compared to mRNA vaccination in a head-to-head comparison. We speculate that specific components of the AZ adenovirus vector may serve as initial trigger(s) of (hyper)inflammation, platelet activation and thrombin generation, potentially lowering the threshold for a cascade of events that both trigger complications related to excessive inflammation, platelet and coagulation activation as observed in epidemiological studies and promote development of VITT when combined with high-titer functionally active PF4 antibodies.
Keywords: COVID-19; TTS; VITT; platelet factor 4; thrombocytopenia; thrombosis; vaccines.
Copyright © 2021 Ostrowski, Søgaard, Tolstrup, Stærke, Lundgren, Østergaard and Hvas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

