Newly developed dual topoisomerase inhibitor P8-D6 is highly active in ovarian cancer
- PMID: 34887943
- PMCID: PMC8649464
- DOI: 10.1177/17588359211059896
Newly developed dual topoisomerase inhibitor P8-D6 is highly active in ovarian cancer
Abstract
Background: Ovarian cancer (OvCa) constitutes a rare and highly aggressive malignancy and is one of the most lethal of all gynaecologic neoplasms. Due to chemotherapy resistance and treatment limitations because of side effects, OvCa is still not sufficiently treatable. Hence, new drugs for OvCa therapy such as P8-D6 with promising antitumour properties have a high clinical need. The benzo[c]phenanthridine P8-D6 is an effective inductor of apoptosis by acting as a dual topoisomerase I/II inhibitor.
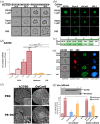
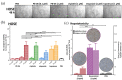
Methods: In the present study, the effectiveness of P8-D6 on OvCa was investigated in vitro. In various OvCa cell lines and ex vivo primary cells, the apoptosis induction compared with standard therapeutic agents was determined in two-dimensional monolayers. Expanded by three-dimensional and co-culture, the P8-D6 treated cells were examined for changes in cytotoxicity, apoptosis rate and membrane integrity via scanning electron microscopy (SEM). Likewise, the effects of P8-D6 on non-cancer human ovarian surface epithelial cells and primary human hepatocytes were determined.
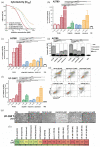
Results: This study shows a significant P8-D6-induced increase in apoptosis and cytotoxicity in OvCa cells which surpasses the efficacy of well-established drugs like cisplatin or the topoisomerase inhibitors etoposide and topotecan. Non-cancer cells were affected only slightly by P8-D6. Moreover, no hepatotoxic effect in in vitro studies was detected.
Conclusion: P8-D6 is a strong and rapid inductor of apoptosis and might be a novel treatment option for OvCa therapy.
Keywords: OvCa; apoptosis; chemotherapy; drug development; dual topoisomerase inhibitor; hepatotoxicity.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Figures

Similar articles
-
High Antitumor Activity of the Dual Topoisomerase Inhibitor P8-D6 in Breast Cancer.Cancers (Basel). 2021 Dec 21;14(1):2. doi: 10.3390/cancers14010002. Cancers (Basel). 2021. PMID: 35008166 Free PMC article.
-
Dual Topoisomerase Inhibitor Is Highly Potent and Improves Antitumor Response to Radiotherapy in Cervical Carcinoma.Int J Mol Sci. 2025 Mar 21;26(7):2829. doi: 10.3390/ijms26072829. Int J Mol Sci. 2025. PMID: 40243435 Free PMC article.
-
The Aza-Analogous Benzo[c]phenanthridine P8-D6 Acts as a Dual Topoisomerase I and II Poison, thus Exhibiting Potent Genotoxic Properties.Molecules. 2020 Mar 27;25(7):1524. doi: 10.3390/molecules25071524. Molecules. 2020. PMID: 32230817 Free PMC article.
-
The Chemoprevention of Ovarian Cancer: the Need and the Options.Curr Pharmacol Rep. 2018;4(3):250-260. doi: 10.1007/s40495-018-0133-6. Epub 2018 May 2. Curr Pharmacol Rep. 2018. PMID: 30363743 Free PMC article. Review.
-
Topotecan - A novel topoisomerase I inhibitor: pharmacology and clinical experience.Oncology. 1999;56(1):1-12. doi: 10.1159/000011923. Oncology. 1999. PMID: 9885371 Review.
Cited by
-
High Antitumor Activity of the Dual Topoisomerase Inhibitor P8-D6 in Breast Cancer.Cancers (Basel). 2021 Dec 21;14(1):2. doi: 10.3390/cancers14010002. Cancers (Basel). 2021. PMID: 35008166 Free PMC article.
-
Combined PARP and Dual Topoisomerase Inhibition Potentiates Genome Instability and Cell Death in Ovarian Cancer.Int J Mol Sci. 2022 Sep 10;23(18):10503. doi: 10.3390/ijms231810503. Int J Mol Sci. 2022. PMID: 36142413 Free PMC article.
-
In Vivo and In Vitro Pharmacokinetic Studies of a Dual Topoisomerase I/II Inhibitor.ACS Pharmacol Transl Sci. 2025 Mar 12;8(4):1050-1071. doi: 10.1021/acsptsci.4c00596. eCollection 2025 Apr 11. ACS Pharmacol Transl Sci. 2025. PMID: 40242581
-
Dual Topoisomerase Inhibitor Is Highly Potent and Improves Antitumor Response to Radiotherapy in Cervical Carcinoma.Int J Mol Sci. 2025 Mar 21;26(7):2829. doi: 10.3390/ijms26072829. Int J Mol Sci. 2025. PMID: 40243435 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30. - PubMed
LinkOut - more resources
Full Text Sources

