Copper chelating protein hydrolysate from Salvia hispanica L. by pepsin-pancreatin treatment
- PMID: 34888529
- PMCID: PMC8636727
- DOI: 10.1016/j.crfs.2021.11.007
Copper chelating protein hydrolysate from Salvia hispanica L. by pepsin-pancreatin treatment
Abstract
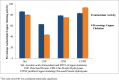
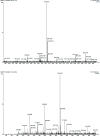
Salvia hispanica L. (Chia) seeds are good source of proteins with diverse health benefits. The seed protein was extracted through alkaline solubilisation followed by acid precipitation to separate fibres and are digested sequentially by pepsin and pancreatin. Enzyme-substrate ratio, temperature and contact time had high impact on degree of hydrolysis affecting their chelating ability. Maximum degree of hydrolysis (14.06%) and maximum copper chelation (74.98%) was obtained at 4% w/w enzyme-substrate ratio at 37 °C for 4 h. Copper chelating enzymatic hydrolysate was isolated by HiTrap chelating column and purified further by rpHPLC. Out of nine fractions obtained by rpHPLC the sixth fraction with 93.09 ± 0.16% of copper chelating activity and 82.91 ± 0.52% of antioxidant activity was further characterized as Copper chelating Chia Protein Hydrolysate (CCPH). Ultraviolet spectroscopy and fluorescence spectroscopic studies revealed the interaction of the major chelating sites of the CCPH with the copper divalent ion. The purified CCPH was subjected to LC-MS/ESI-TOF analysis from which six major intense peaks obtained with m/z value ranging from 0.4 kDa to 2.5 kDa were identified and sequenced using Mascot database. The functional behaviour and the binding capacity of these peptides were analysed by their amino acid composition. The CCPH was stable in a simulated gastric condition and its chelating ability remained unaltered. These results explored an informative bioactive peptides with varied activity and one valuable among is the copper chelating with antioxidant property. Furthermore, these Chia seed protein hydrolysates can be useful as dietary supplements to enhance mineral bioavailability.
Keywords: Chia seed; Copper chelating peptides; Protein hydrolysate.
© 2021 Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no competing financial interests and personal relationship that could have appeared to influence the work reported in this paper.
Figures






References
-
- Budseekoad S., Yupanqui C.T., Sirinupong N., Alashi A.M., Aluko R.E., Youravong W. Structural and functional characterization of calcium and iron-binding peptides from mung bean protein hydrolysate. J. Funct. Foods. 2018;49:333–341. doi: 10.1016/j.jff.2018.07.041. - DOI
-
- Chim-Chi Y., Gallegos-Tintore S., Jimenez-Martínez C., Davila-Ortiz G., Chel-Guerrero L. Antioxidant capacity of Mexican chia (Salvia hispanica L.) protein hydrolyzates. J. Food Meas. Char. 2017;12(1):323–331. doi: 10.1007/s11694-017-9644-9. - DOI
-
- Church F.C., Swaisgood H.E., Porter D.H., Catignani G.L. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 1983;66(6):1219–1227. doi: 10.3168/jds.S0022-0302(83)81926-2. - DOI
LinkOut - more resources
Full Text Sources

