Reduced Magnitude and Durability of Humoral Immune Responses to COVID-19 mRNA Vaccines Among Older Adults
- PMID: 34888688
- PMCID: PMC8689804
- DOI: 10.1093/infdis/jiab592
Reduced Magnitude and Durability of Humoral Immune Responses to COVID-19 mRNA Vaccines Among Older Adults
Abstract
Background: The magnitude and durability of immune responses to coronavirus disease 2019 (COVID-19) mRNA vaccines remain incompletely characterized in the elderly.
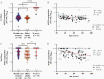
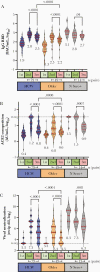
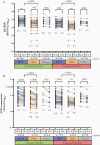
Methods: Anti-spike receptor-binding domain (RBD) antibodies, angiotensin-converting enzyme 2 (ACE2) competition, and virus neutralizing activities were assessed in plasma from 151 health care workers and older adults (range, 24-98 years of age) 1 month following the first vaccine dose, and 1 and 3 months following the second dose.
Results: Older adults exhibited significantly weaker responses than younger health care workers for all humoral measures evaluated and at all time points tested, except for ACE2 competition activity after 1 vaccine dose. Moreover, older age remained independently associated with weaker responses even after correction for sociodemographic factors, chronic health condition burden, and vaccine-related variables. By 3 months after the second dose, all humoral responses had declined significantly in all participants, and remained significantly lower among older adults, who also displayed reduced binding antibodies and ACE2 competition activity towards the Delta variant.
Conclusions: Humoral responses to COVID-19 mRNA vaccines are significantly weaker in older adults, and antibody-mediated activities in plasma decline universally over time. Older adults may thus remain at elevated risk of infection despite vaccination.
Keywords: COVID-19; antibodies; humoral responses; mRNA vaccine; older adults; viral neutralization.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

