Astroglial Serotonin Receptors as the Central Target of Classic Antidepressants
- PMID: 34888840
- PMCID: PMC9015684
- DOI: 10.1007/978-3-030-77375-5_13
Astroglial Serotonin Receptors as the Central Target of Classic Antidepressants
Abstract
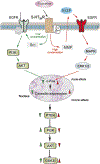
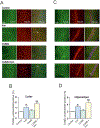
Major depressive disorder (MDD) presents multiple clinical phenotypes and has complex underlying pathological mechanisms. Existing theories cannot completely explain the pathophysiological mechanism(s) of MDD, while the pharmacology of current antidepressants is far from being fully understood. Astrocytes, the homeostatic and defensive cells of the central nervous system, contribute to shaping behaviors, and regulating mood and emotions. A detailed introduction on the role of astrocytes in depressive disorders is thus required, to which this chapter is dedicated. We also focus on the interactions between classic antidepressants and serotonin receptors, overview the role of astrocytes in the pharmacological mechanisms of various antidepressants, and present astrocytes as targets for the treatment of bipolar disorder. We provide a foundation of knowledge on the role of astrocytes in depressive disorders and astroglial 5-HT2B receptors as targets for selective serotonin reuptake inhibitors in vivo and in vitro.
Keywords: 5-HT2B receptors; Astrocytes; Fluoxetine; Major depressive disorder; Selective serotonin reuptake inhibitors.
© 2021. The Author(s), under exclusive license to Springer Nature Switzerland AG.
Figures

Similar articles
-
The Association Between Antidepressant Effect of SSRIs and Astrocytes: Conceptual Overview and Meta-analysis of the Literature.Neurochem Res. 2021 Oct;46(10):2731-2745. doi: 10.1007/s11064-020-03225-6. Epub 2021 Feb 1. Neurochem Res. 2021. PMID: 33527219
-
Altered γ-aminobutyric acid neurotransmission in major depressive disorder: a critical review of the supporting evidence and the influence of serotonergic antidepressants.Drug Des Devel Ther. 2015 Jan 19;9:603-24. doi: 10.2147/DDDT.S62912. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25653499 Free PMC article. Review.
-
Modulating the serotonin system in the treatment of major depressive disorder.CNS Spectr. 2014 Dec;19 Suppl 1:57-67; quiz 54-7, 68. doi: 10.1017/S1092852914000613. CNS Spectr. 2014. PMID: 25544378 Review.
-
Vortioxetine: a New Treatment for Major Depressive Disorder.Expert Opin Pharmacother. 2016;17(3):421-31. doi: 10.1517/14656566.2016.1133588. Expert Opin Pharmacother. 2016. PMID: 26679430 Review.
-
[Efficacy of atypical antipsychotics in depressive syndromes].Encephale. 2004 Nov-Dec;30(6):583-9. doi: 10.1016/s0013-7006(04)95474-7. Encephale. 2004. PMID: 15738862 Review. French.
Cited by
-
Major depressive disorder: hypothesis, mechanism, prevention and treatment.Signal Transduct Target Ther. 2024 Feb 9;9(1):30. doi: 10.1038/s41392-024-01738-y. Signal Transduct Target Ther. 2024. PMID: 38331979 Free PMC article. Review.
-
Astrocytes in human central nervous system diseases: a frontier for new therapies.Signal Transduct Target Ther. 2023 Oct 13;8(1):396. doi: 10.1038/s41392-023-01628-9. Signal Transduct Target Ther. 2023. PMID: 37828019 Free PMC article. Review.
-
Astrocyte Heterogeneity in Regulation of Synaptic Activity.Cells. 2022 Oct 5;11(19):3135. doi: 10.3390/cells11193135. Cells. 2022. PMID: 36231097 Free PMC article. Review.
-
Dysfunctional serotonergic neuron-astrocyte signaling in depressive-like states.Mol Psychiatry. 2023 Sep;28(9):3856-3873. doi: 10.1038/s41380-023-02269-8. Epub 2023 Sep 29. Mol Psychiatry. 2023. PMID: 37773446 Free PMC article.
-
Astrocyte regulation of synaptic signaling in psychiatric disorders.Neuropsychopharmacology. 2023 Jan;48(1):21-36. doi: 10.1038/s41386-022-01338-w. Epub 2022 May 16. Neuropsychopharmacology. 2023. PMID: 35577914 Free PMC article. Review.
References
-
- Adrien J, Tissier MH, Lanfumey L, Haj-Dahmane S, Jolas T, Franc B, Hamon M (1992) Central action of 5-HT3 receptor ligands in the regulation of sleep-wakefulness and raphe neuronal activity in the rat. Neuropharmacology 31:519–529 - PubMed
-
- Albert PR, Lemonde S (2004) 5-HT1A receptors, gene repression, and depression: guilt by association. Neuroscientist 10:575–593 - PubMed
-
- Albinsson A, Björk A, Svartengren J, Klint T, Andersson G (1994) Preclinical pharmacology of FG5893: a potential anxiolytic drug with high affinity for both 5-HT1A and 5-HT2A receptors. Eur J Pharmacol 261:285–294 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
