Introduction to Hemodynamic Forces Analysis: Moving Into the New Frontier of Cardiac Deformation Analysis
- PMID: 34889114
- PMCID: PMC9075239
- DOI: 10.1161/JAHA.121.023417
Introduction to Hemodynamic Forces Analysis: Moving Into the New Frontier of Cardiac Deformation Analysis
Abstract
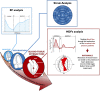
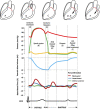
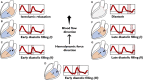
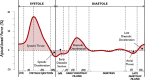
The potential relevance of blood flow for describing cardiac function has been known for the past 2 decades, but the association of clinical parameters with the complexity of fluid motion is still not well understood. Hemodynamic force (HDF) analysis represents a promising approach for the study of blood flow within the ventricular chambers through the exploration of intraventricular pressure gradients. Previous experimental studies reported the significance of invasively measured cardiac pressure gradients in patients with heart failure. Subsequently, advances in cardiovascular imaging allowed noninvasive assessment of pressure gradients during progression and resolution of ventricular dysfunction and in the setting of resynchronization therapy. The HDF analysis can amplify mechanical abnormalities, detect them earlier compared with conventional ejection fraction and strain analysis, and possibly predict the development of cardiac remodeling. Alterations in HDFs provide the earliest signs of impaired cardiac physiology and can therefore transform the existing paradigm of cardiac function analysis once implemented in routine clinical care. Until recently, the HDF investigation was possible only with contrast-enhanced echocardiography and magnetic resonance imaging, precluding its widespread clinical use. A mathematical model, based on the first principle of fluid dynamics and validated using 4-dimensional-flow-magnetic resonance imaging, has allowed HDF analysis through routine transthoracic echocardiography, making it more readily accessible for routine clinical use. This article describes the concept of HDF analysis and reviews the existing evidence supporting its application in several clinical settings. Future studies should address the prognostic importance of HDF assessment in asymptomatic patients and its incorporation into clinical decision pathways.
Keywords: blood flow; cardiac mechanics; deformation imaging; heart failure; intraventricular pressure gradient.
Figures



Similar articles
-
Introduction to hemodynamic forces by echocardiography.Int J Cardiol. 2023 Jan 1;370:442-444. doi: 10.1016/j.ijcard.2022.11.019. Epub 2022 Nov 14. Int J Cardiol. 2023. PMID: 36395921
-
Cardiac fluid dynamics meets deformation imaging.Cardiovasc Ultrasound. 2018 Feb 20;16(1):4. doi: 10.1186/s12947-018-0122-2. Cardiovasc Ultrasound. 2018. PMID: 29458381 Free PMC article. Review.
-
Hemodynamic Force Based on Cardiac Magnetic Resonance Imaging: State of the Art and Perspective.J Magn Reson Imaging. 2025 Mar;61(3):1033-1047. doi: 10.1002/jmri.29483. Epub 2024 Jul 3. J Magn Reson Imaging. 2025. PMID: 38958118 Review.
-
On estimating intraventricular hemodynamic forces from endocardial dynamics: A comparative study with 4D flow MRI.J Biomech. 2017 Jul 26;60:203-210. doi: 10.1016/j.jbiomech.2017.06.046. Epub 2017 Jul 5. J Biomech. 2017. PMID: 28711164
-
Assessing cardiac mechanics through left ventricular haemodynamic forces.Eur Heart J Imaging Methods Pract. 2024 Jul 25;2(3):qyae077. doi: 10.1093/ehjimp/qyae077. eCollection 2024 Jul. Eur Heart J Imaging Methods Pract. 2024. PMID: 39224620 Free PMC article. Review.
Cited by
-
Cardiac imaging in athlete's heart: current status and future prospects.Cardiovasc Ultrasound. 2023 Dec 14;21(1):21. doi: 10.1186/s12947-023-00319-3. Cardiovasc Ultrasound. 2023. PMID: 38098064 Free PMC article. Review.
-
Acute Modification of Hemodynamic Forces in Patients with Severe Aortic Stenosis after Transcatheter Aortic Valve Implantation.J Clin Med. 2023 Feb 3;12(3):1218. doi: 10.3390/jcm12031218. J Clin Med. 2023. PMID: 36769866 Free PMC article.
-
Haemodynamic Forces: Emerging Markers of Ventricular Remodelling in Multiple Myeloma Cardiovascular Baseline Risk Assessment.Cancers (Basel). 2024 Sep 4;16(17):3081. doi: 10.3390/cancers16173081. Cancers (Basel). 2024. PMID: 39272939 Free PMC article.
-
Intraventricular pressure difference estimation based on blood speckle tracking-invasive validation and early clinical application.Eur Heart J Cardiovasc Imaging. 2025 Jul 31;26(8):1346-1357. doi: 10.1093/ehjci/jeaf149. Eur Heart J Cardiovasc Imaging. 2025. PMID: 40365693 Free PMC article.
-
A scenario for heart failure during the filling phase.Sci Rep. 2024 Oct 1;14(1):22760. doi: 10.1038/s41598-024-74155-4. Sci Rep. 2024. PMID: 39354060 Free PMC article.
References
-
- Eriksson J, Bolger AF, Ebbers T, Carlhall CJ. Left ventricular hemodynamic forces are altered in patients with dilated cardiomyopathy. J Cardiovasc Magn Reson. 2015;17:P292. doi: 10.1186/1532-429X-17-S1-P282 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

