Factor-mimetic and rebalancing therapies in hemophilia A and B: the end of factor concentrates?
- PMID: 34889356
- PMCID: PMC8791123
- DOI: 10.1182/hematology.2021000253
Factor-mimetic and rebalancing therapies in hemophilia A and B: the end of factor concentrates?
Abstract
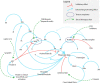
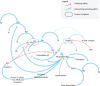
Hemophilia A (HA) and B are inherited bleeding disorders caused by a deficiency of factor VIII or factor IX, respectively. The current standard of care is the administration of recombinant or purified factor. However, this treatment strategy still results in a high economic and personal burden to patients, which is further exacerbated by the development of inhibitors-alloantibodies to factor. The treatment landscape is changing, with nonfactor therapeutics playing an increasing role in what we consider to be the standard of care. Emicizumab, a bispecific antibody that mimics the function of factor VIIIa, is the first such nonfactor therapy to gain US Food and Drug Administration approval and is rapidly changing the paradigm for HA treatment. Other therapies on the horizon seek to target anticoagulant proteins in the coagulation cascade, thus "rebalancing" a hemorrhagic tendency by introducing a thrombotic tendency. This intricate hemostatic balancing act promises great things for patients in need of more treatment options, but are these other therapies going to replace factor therapy? In light of the many challenges facing these therapies, should they be viewed as a replacement of our current standard of care? This review discusses the background, rationale, and potential of nonfactor therapies as well as the anticipated pitfalls and limitations. This is done in the context of a review of our current understanding of the many aspects of the coagulation system.
Copyright © 2021 by The American Society of Hematology.
Conflict of interest statement
Patrick Ellsworth: Is an NHF-Takeda Clinical Research Fellowship award recipient.
Alice Ma: research funding and honoraria: Takeda.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

