Have we reached a molecular era in myelodysplastic syndromes?
- PMID: 34889424
- PMCID: PMC8791166
- DOI: 10.1182/hematology.2021000276
Have we reached a molecular era in myelodysplastic syndromes?
Abstract
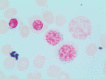
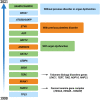
Myelodysplastic syndromes (MDS) are characterized by heterogeneous biological and clinical characteristics, leading to variable outcomes. The availability of sophisticated platforms of genome sequencing allowed the discovery of recurrently mutated genes, which have led to a new era in MDS. This is reflected by the 2016 update of the World Health Organization classification, in which the criteria to define MDS with ringed sideroblasts include the presence of SF3B1 mutations. Further, the detection of somatic mutations in myeloid genes at high variant allele frequency guides the diagnostic algorithm in cases with cytopenias, unclear dysplastic changes, and normal karyotypes, supporting MDS over alternative diagnoses. SF3B1 mutations have been shown to play a positive prognostic role, while mutations in ASXL1, EZH2, RUNX1, and TP53 have been associated with a dismal prognosis. This is particularly relevant in lower- and intermediate-risk disease, in which a higher number of mutations and/or the presence of "unfavorable" somatic mutations may support the use of disease-modifying treatments. In the near future, the incorporation of mutation profiles in currently used prognostication systems, also taking into consideration the classical patient clinical variables (including age and comorbidities), will support a more precise disease stratification, eg, the assignment to targeted treatment approaches or to allogeneic stem cell transplantation in younger patients.
Copyright © 2021 by The American Society of Hematology.
Conflict of interest statement
Maria Teresa Voso: no competing financial interests to declare.
Carmelo Gurnari: no competing financial interests to declare.
Figures




References
-
- Greenberg P, Cox C, LeBeau MM, et al.. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079-2088. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

