JMM Profile: Carbapenems: a broad-spectrum antibiotic
- PMID: 34889726
- PMCID: PMC8744278
- DOI: 10.1099/jmm.0.001462
JMM Profile: Carbapenems: a broad-spectrum antibiotic
Abstract
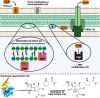
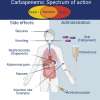
Carbapenems are potent members of the β-lactam family that inhibit bacterial cell-wall biosynthesis inhibitors . They are highly effective against Gram-negative and Gram-positive drug-resistant infections . As such, carbapenems are typically reserved as an antibiotic of last resort. The WHO lists meropenem as an essential medicine. Nausea and vomiting are reported in ≤20% of carbapenem recipients, with 1.5% suffering seizures. Enzymatic hydrolysis of the β-lactam ring is the main driver of clinical resistance. These enzymes can be classified as Class A, B and D. Classes A and D are serine β-lactamases, whereas Class B rely on metal-mediated hydrolysis, typically through zinc.
Keywords: antimicrobial resistance; last resort; β-lactams.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
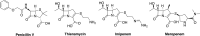
References
-
- Trebosc V, Schellhorn B, Schill J, Lucchini V, Bühler J, et al. In vitro activity of rifabutin against 293 contemporary carbapenem-resistant Acinetobacter baumannii clinical isolates and characterization of rifabutin mode of action and resistance mechanisms. J Antimicrob Chemother. 2020;75:3552–3562. doi: 10.1093/jac/dkaa370. - DOI - PMC - PubMed
-
- Yocum RR, Rasmussen JR, Strominger JL. The mechanism of action of penicillin. Penicillin acylates the active site of Bacillus stearothermophilus D-alanine carboxypeptidase. J Biol Chem. 1980;255:3977–3986. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

