NASHFit: A randomized controlled trial of an exercise training program to reduce clotting risk in patients with NASH
- PMID: 34890063
- PMCID: PMC9184303
- DOI: 10.1002/hep.32274
NASHFit: A randomized controlled trial of an exercise training program to reduce clotting risk in patients with NASH
Abstract
Background and aims: NASH is a common disease associated with increased rates of thromboembolism (TE). Although exercise training can lessen thrombotic risk in patients with vascular disease, whether similar findings are observed in patients with NASH is open for study.
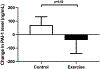
Approach and results: We conducted a 20-week randomized controlled clinical trial involving patients with biopsy-confirmed NASH. Patients were randomly assigned (2:1 ratio) to receive either an exercise training program or standard clinical care. The primary endpoint was change in plasminogen activator inhibitor 1 (PAI-1) level, an established thrombotic biomarker. Twenty-eight patients were randomly assigned (18 exercise training and 10 standard clinical care). PAI-1 level was significantly decreased by exercise training when compared to standard clinical care (-40 ± 100 vs. +70 ± 63 ng/ml; p = 0.02). Exercise training decreased MRI proton density fat fraction (MRI-PDFF; -4.7 ± 5.6 vs. 1.2 ± 2.8% absolute liver fat; p = 0.01); 40% of exercise subjects had a ≥30% relative reduction in MRI-PDFF (histological response threshold) compared to 13% for standard of care (p < 0.01). Exercise training improved fitness (VO2 peak, +3.0 ± 5.6 vs. -1.8 ± 5.1 ml/kg/min; p = 0.05) in comparison to standard clinical care.
Conclusions: This clinical trial showed that, independent of weight loss or dietary change, exercise training resulted in a significantly greater decrease in thrombotic risk than standard clinical care in patients with NASH, in parallel with MRI-PDFF reduction and improvement in fitness. Future studies are required to determine whether exercise training can directly impact patient outcomes and lower rates of TE.
© 2022 American Association for the Study of Liver Diseases.
Figures



Comment in
-
Improving the fitness of the NASH clinical trial: Standardizing the standard-of-care intervention.Hepatology. 2022 Jul;76(1):12-14. doi: 10.1002/hep.32369. Epub 2022 Mar 19. Hepatology. 2022. PMID: 35092072 No abstract available.
-
Letter to the editor: Is PAI-1 a thrombotic biomarker in NASH cirrhosis?Hepatology. 2022 Jul;76(1):E16-E17. doi: 10.1002/hep.32392. Epub 2022 Feb 17. Hepatology. 2022. PMID: 35112379 No abstract available.
References
-
- Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol 2019;70:531–544. - PubMed
-
- Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, et al. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

