A randomized placebo-controlled phase 3 study of mesenchymal stem cells induced to secrete high levels of neurotrophic factors in amyotrophic lateral sclerosis
- PMID: 34890069
- PMCID: PMC9305113
- DOI: 10.1002/mus.27472
A randomized placebo-controlled phase 3 study of mesenchymal stem cells induced to secrete high levels of neurotrophic factors in amyotrophic lateral sclerosis
Erratum in
-
Erratum.Muscle Nerve. 2022 Oct;66(4):E26-E27. doi: 10.1002/mus.27697. Epub 2022 Aug 12. Muscle Nerve. 2022. PMID: 35960989 Free PMC article. No abstract available.
Abstract
Introduction/aims: Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative illness with great unmet patient need. We aimed to evaluate whether mesenchymal stem cells induced to secrete high levels of neurotrophic factors (MSC-NTF), a novel autologous cell-therapy capable of targeting multiple pathways, could safely slow ALS disease progression.
Methods: This randomized, double-blind, placebo-controlled study enrolled ALS participants meeting revised El Escorial criteria, revised ALS Functional Rating Scale (ALSFRS-R) ≥25 (screening) and ≥3 ALSFRS-R points decline prior to randomization. Participants received three treatments of MSC-NTF or placebo intrathecally. The primary endpoint evaluated efficacy of MSC-NTF through a responder analysis and safety. A change in disease progression post-treatment of ≥1.25 points/mo defines a clinical response. A pre-specified analysis leveraged baseline ALSFRS-R of 35 as a subgroup threshold.
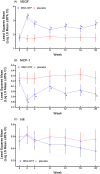
Results: Overall, MSC-NTF treatment was well tolerated; there were no safety concerns. Thirty-three percent of MSC-NTF and 28% of placebo participants met clinical response criteria at 28 wk (odds ratio [OR] = 1.33, P = .45); thus, the primary endpoint was not met. A pre-specified analysis of participants with baseline ALSFRS-R ≥ 35 (n = 58) showed a clinical response rate at 28 wk of 35% MSC-NTF and 16% placebo (OR = 2.6, P = .29). Significant improvements in cerebrospinal biomarkers of neuroinflammation, neurodegeneration, and neurotrophic factor support were observed with MSC-NTF, with placebo unchanged.
Discussion: The study did not reach statistical significance on the primary endpoint. However, a pre-specified subgroup suggests that MSC-NTF participants with less severe disease may have retained more function compared to placebo. Given the unmet patient need, the results of this trial warrant further investigation.
Keywords: ALSFRS-R; amyotrophic lateral sclerosis; biomarker; clinical trial; stem cells.
© 2021 BrainStorm Cell Therapeutics. Muscle & Nerve published by Wiley Periodicals LLC.
Conflict of interest statement
D.A.B. reports personal fees from Brainstorm Cell Therapeutics. J.D.B. reports personal fees from Biogen, Clene Nanomedicine, MT Pharma of America, MT Pharma Holdings of America, Janssen; grants from Alexion, Biogen, Amylyx Therapeutics, MT Pharma of America, Anelixis Therapeutics, Genentech, Rapa Therapeutics, MT Pharma Holdings of America, nQ Medical, NINDS, Muscular Dystrophy Association, ALS One, ALS Association, ALS Finding A Cure, Rapa Therapeutics; and has equity in ReactNeuro. J.K. reports consulting fees from MT Pharma, Denali Therapeutics, Biogen, Genetech, Amylyx, Cytokinetics, Wave, and Calico. K.N. reports personal fees from Alector LLC, AI Therapeutics, Biogen, Biohaven, MT Pharma, Wave Therapeutics, Enclear, and Regeneron; grants from ALS Association, Muscular Dystrophy Association, ALS Finding a Cure, and Target ALS. M.C. reports personal fees from Lilly Avexis, Pontifax, Orion, MT Pharma, Denali, Biogen, Pharmnext, Treeway, Revalasio, Takeda, Aclipse, Biohaven, Sunovian, Anelixis, Disarm, ALS Biopharma, Cytokinetics, RRD, Immunity Pharma, Helixsmith, Wave, Transposon, Quralis, Faze, Regeneron, and AB Sciences. N.A.G. received research support from Alexion, Brainstorm Cell Therapeutics, Cytokinetics, Fulcrum, Healey Platform, Kezar, Medicinova, Octapharma, Orion, Orphazyme; served on Advisory Boards for Acceleron, Alexion, Argenx, CSL Behring, MT Pharma, Sanofi Genzyme, Sarepta, UCB; received travel reimbursement and honoraria; and served on the speaker's bureau for CSL. N.P.S. reports research support from the National Institutes of Health (R01 CA21887), Regenerative Medicine Minnesota, Target ALS, and ALS Association. R.H.B. Jr. reports personal fees from Wave Lifesciences and serves on advisory boards of ALS Finding a Cure, Project ALS, and NEALS. R.L. received research support from Argenx, Annexon, Biotest, CSL Behring, Grifols, Pharnext, Sanofi‐Genzyme, Seattle Genetics, UCB, and Pfizer; honoraria from Medscape, Alnylam, Akcea; and served on advisory boards for GBS‐CIDP Foundation International, Myasthenia Gravis Foundation of America, MGF of California, and Board of Directors for Peripheral Nerve Society. S.R.L., R.K., Y.G, Y.S.L., and R.A are employees of and hold stock in Brainstorm Cell Therapeutics. A.J.W., L.J., M.A.O., M.B., R.M., and T.M. have no conflicts of interest to report.
Figures