A single motor neuron determines the rhythm of early motor behavior in Ciona
- PMID: 34890229
- PMCID: PMC8664258
- DOI: 10.1126/sciadv.abl6053
A single motor neuron determines the rhythm of early motor behavior in Ciona
Abstract
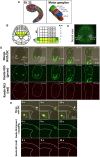
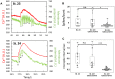
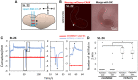
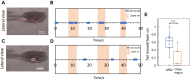
Recent work in tunicate supports the similarity between the motor circuits of vertebrates and basal deuterostome lineages. To understand how the rhythmic activity in motor circuits is acquired during development of protochordate Ciona, we investigated the coordination of the motor response by identifying a single pair of oscillatory motor neurons (MN2/A10.64). The MN2 neurons had Ca2+ oscillation with an ~80-s interval that was cell autonomous even in a dissociated single cell. The Ca2+ oscillation of MN2 coincided with the early tail flick (ETF). The spikes of the membrane potential in MN2 gradually correlated with the rhythm of ipsilateral muscle contractions in ETFs. The optogenetic experiments indicated that MN2 is a necessary and sufficient component of ETFs. These results indicate that MN2 is indispensable for the early spontaneous rhythmic motor behavior of Ciona. Our findings shed light on the understanding of development and evolution of chordate rhythmical locomotion.
Figures




Similar articles
-
A single oscillating proto-hypothalamic neuron gates taxis behavior in the primitive chordate Ciona.Curr Biol. 2023 Aug 21;33(16):3360-3370.e4. doi: 10.1016/j.cub.2023.06.080. Epub 2023 Jul 24. Curr Biol. 2023. PMID: 37490920 Free PMC article.
-
Transitions of motor neuron activities during Ciona development.Front Cell Dev Biol. 2023 Jan 13;11:1100887. doi: 10.3389/fcell.2023.1100887. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36711039 Free PMC article.
-
MiniSOG2-mediated Specific Photoablation of Motor Neurons in Ascidian Embryos.Bio Protoc. 2022 Oct 20;12(20):e4537. doi: 10.21769/BioProtoc.4537. eCollection 2022 Oct 20. Bio Protoc. 2022. PMID: 36353720 Free PMC article.
-
Development, functional organization, and evolution of vertebrate axial motor circuits.Neural Dev. 2018 Jun 1;13(1):10. doi: 10.1186/s13064-018-0108-7. Neural Dev. 2018. PMID: 29855378 Free PMC article. Review.
-
New perspectives on the evolution of protochordate sensory and locomotory systems, and the origin of brains and heads.Philos Trans R Soc Lond B Biol Sci. 2001 Oct 29;356(1414):1565-72. doi: 10.1098/rstb.2001.0974. Philos Trans R Soc Lond B Biol Sci. 2001. PMID: 11604123 Free PMC article. Review.
Cited by
-
A single oscillating proto-hypothalamic neuron gates taxis behavior in the primitive chordate Ciona.bioRxiv [Preprint]. 2023 Jun 3:2023.04.24.538092. doi: 10.1101/2023.04.24.538092. bioRxiv. 2023. Update in: Curr Biol. 2023 Aug 21;33(16):3360-3370.e4. doi: 10.1016/j.cub.2023.06.080. PMID: 37162881 Free PMC article. Updated. Preprint.
-
Motor neurons in the tunicate caudal central nervous system reveal homology to the vertebrate spinal cord.Development. 2025 Mar 1;152(5):DEV204525. doi: 10.1242/dev.204525. Epub 2025 Mar 13. Development. 2025. PMID: 40079869
-
Development and circuitry of the tunicate larval Motor Ganglion, a putative hindbrain/spinal cord homolog.J Exp Zool B Mol Dev Evol. 2024 May;342(3):200-211. doi: 10.1002/jez.b.23221. Epub 2023 Sep 7. J Exp Zool B Mol Dev Evol. 2024. PMID: 37675754 Free PMC article. Review.
-
Deep homologies in chordate caudal central nervous systems.bioRxiv [Preprint]. 2024 Jun 4:2024.06.03.597227. doi: 10.1101/2024.06.03.597227. bioRxiv. 2024. PMID: 38895365 Free PMC article. Preprint.
-
A single oscillating proto-hypothalamic neuron gates taxis behavior in the primitive chordate Ciona.Curr Biol. 2023 Aug 21;33(16):3360-3370.e4. doi: 10.1016/j.cub.2023.06.080. Epub 2023 Jul 24. Curr Biol. 2023. PMID: 37490920 Free PMC article.
References
-
- Muntz L., Myogenesis in the trunk and leg during development of the tadpole of Xenopus laevis (Daudin 1802). J. Embryol. Exp. Morphol. 33, 757–774 (1975). - PubMed
-
- Saint-Amant L., Drapeau P., Time course of the development of motor behaviors in the zebrafish embryo. J. Neurobiol. 37, 622–632 (1998). - PubMed
-
- Wenner P., O’Donovan M. J., Mechanisms that initiate spontaneous network activity in the developing chick spinal cord. J. Neurophysiol. 86, 1481–1498 (2001). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

