Oncologic Treatment of HIV-Associated Kaposi Sarcoma 40 Years on
- PMID: 34890242
- PMCID: PMC8769148
- DOI: 10.1200/JCO.21.02040
Oncologic Treatment of HIV-Associated Kaposi Sarcoma 40 Years on
Abstract
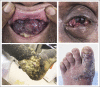
The observation in 1981 of the emergence of Kaposi sarcoma (KS) among young men who had sex with men was one of the first harbingers of the HIV epidemic. With advances in HIV care, the incidence of HIV-associated KS (HIV+KS) has decreased over time in the United States. However, it remains a persistent malignancy among some HIV-infected populations and is one of the most common tumors in sub-Saharan Africa. Because of the relapsing and remitting nature of this cancer, patients with HIV+KS can experience significant, long-term, morbidity. Patients with severe HIV+KS may also have concurrent lymphoproliferative syndromes, malignancies, and/or infections that can contribute to mortality. Several chemotherapy agents were explored in clinical trials for HIV+KS during the early stage of the epidemic. As HIV+KS emerges with CD4 lymphopenia and immunodysregulation, T-cell-sparing options are important to consider. Here, we explore the pathogenesis of HIV+KS and the current evidence for immunotherapy and therapies that potentially target KS pathogenesis. This review provides the current landscape of therapies for HIV+KS and highlights management issues for patients with HIV and cancer.
Conflict of interest statement
Figures

References
-
- Centers for Disease Control (CDC) : Kaposi's sarcoma and Pneumocystis pneumonia among homosexual men—New York City and California. MMWR Morb Mortal Wkly Rep 30:305-308, 1981 - PubMed
-
- Chang Y, Cesarman E, Pessin MS, et al. : Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 266:1865-1869, 1994 - PubMed
-
- Nador RG, Cesarman E, Chadburn A, et al. : Primary effusion lymphoma: A distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood 88:645-656, 1996 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

