Intranasal priming induces local lung-resident B cell populations that secrete protective mucosal antiviral IgA
- PMID: 34890255
- PMCID: PMC8762609
- DOI: 10.1126/sciimmunol.abj5129
Intranasal priming induces local lung-resident B cell populations that secrete protective mucosal antiviral IgA
Abstract
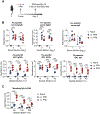
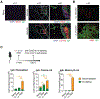
Antibodies secreted at the mucosal surface play an integral role in immune defense by serving to neutralize the pathogen and promote its elimination at the site of entry. Secretory immunoglobulin A (IgA) is a predominant Ig isotype at mucosal surfaces whose epithelial cells express polymeric Ig receptor capable of transporting dimeric IgA to the lumen. Although the role of IgA in intestinal mucosa has been extensively studied, the cell types responsible for secreting the IgA that protects the host against pathogens in the lower respiratory tract are less clear. Here, using a mouse model of influenza virus infection, we demonstrate that intranasal, but not systemic, immunization induces local IgA secretion in the bronchoalveolar space. Using single-cell RNA sequencing, we found a heterogeneous population of IgA-expressing cells within the respiratory mucosa, including tissue-resident memory B cells, plasmablasts, and plasma cells. IgA-secreting cell establishment within the lung required CXCR3. An intranasally administered protein-based vaccine also led to the establishment of IgA-secreting cells in the lung, but not when given intramuscularly or intraperitoneally. Last, local IgA secretion correlated with superior protection against secondary challenge with homologous and heterologous virus infection than circulating antibodies alone. These results provide key insights into establishment of protective immunity in the lung based on tissue-resident IgA-secreting B cells and inform vaccine strategies designed to elicit highly effective immune protection against respiratory virus infections.
Conflict of interest statement
Figures




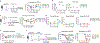
References
-
- Iwasaki A, Exploiting Mucosal Immunity for Antiviral Vaccines. Annu Rev Immunol 34, 575–608 (2016). - PubMed
-
- Macpherson AJ, Yilmaz B, Limenitakis JP, Ganal-Vonarburg SC, IgA Function in Relation to the Intestinal Microbiota. Annu Rev Immunol 36, 359–381 (2018). - PubMed
-
- Kaetzel CS, Robinson JK, Lamm ME, Epithelial transcytosis of monomeric IgA and IgG cross-linked through antigen to polymeric IgA. A role for monomeric antibodies in the mucosal immune system. J Immunol 152, 72–76 (1994). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

