Differential effects of obesity on visceral versus subcutaneous adipose arteries: role of shear-activated Kir2.1 and alterations to the glycocalyx
- PMID: 34890278
- PMCID: PMC8742723
- DOI: 10.1152/ajpheart.00399.2021
Differential effects of obesity on visceral versus subcutaneous adipose arteries: role of shear-activated Kir2.1 and alterations to the glycocalyx
Abstract
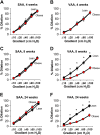
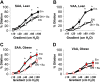
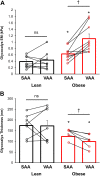
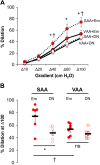
Obesity imposes well-established deficits to endothelial function. We recently showed that obesity-induced endothelial dysfunction was mediated by disruption of the glycocalyx and a loss of Kir channel flow sensitivity. However, obesity-induced endothelial dysfunction is not observed in all vascular beds: visceral adipose arteries (VAAs), but not subcutaneous adipose arteries (SAAs), exhibit endothelial dysfunction. To determine whether differences in SAA versus VAA endothelial function observed in obesity are attributed to differential impairment of Kir channels and alterations to the glycocalyx, mice were fed a normal rodent diet, or a high-fat Western diet to induce obesity. Flow-induced vasodilation (FIV) was measured ex vivo. Functional downregulation of endothelial Kir2.1 was accomplished by transducing adipose arteries from mice and obese humans with adenovirus containing a dominant-negative Kir2.1 construct. Kir function was tested in freshly isolated endothelial cells seeded in a flow chamber for electrophysiological recordings under fluid shear. Atomic force microscopy was used to assess biophysical properties of the glycocalyx. Endothelial dysfunction was observed in VAAs of obese mice and humans. Downregulating Kir2.1 blunted FIV in SAAs, but had no effect on VAAs, from obese mice and humans. Obesity abolished Kir shear sensitivity in VAA endothelial cells and significantly altered the VAA glycocalyx. In contrast, Kir shear sensitivity was observed in SAA endothelial cells from obese mice and effects on SAA glycocalyx were less pronounced. We reveal distinct differences in Kir function and alterations to the glycocalyx that we propose contribute to the dichotomy in SAA versus VAA endothelial function with obesity.NEW & NOTEWORTHY We identified a role for endothelial Kir2.1 in the differences observed in VAA versus SAA endothelial function with obesity. The endothelial glycocalyx, a regulator of Kir activation by shear, is unequally perturbed in VAAs as compared with SAAs, which we propose results in a near complete loss of VAA endothelial Kir shear sensitivity and endothelial dysfunction. We propose that these differences underly the preserved endothelial function of SAA in obese mice and humans.
Keywords: Kir2.1; adipose; endothelial dysfunction; glycocalyx; obesity.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

References
-
- Grizelj I, Cavka A, Bian JT, Szczurek M, Robinson A, Shinde S, Nguyen V, Braunschweig C, Wang E, Drenjancevic I, Phillips SA. Reduced flow-and acetylcholine-induced dilations in visceral compared to subcutaneous adipose arterioles in human morbid obesity. Microcirculation 22: 44–53, 2015. doi:10.1111/micc.12164. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

