Mechanistic characterization of endothelial sprouting mediated by pro-angiogenic signaling
- PMID: 34890488
- PMCID: PMC9285777
- DOI: 10.1111/micc.12744
Mechanistic characterization of endothelial sprouting mediated by pro-angiogenic signaling
Abstract
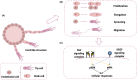
Objective: We aim to quantitatively characterize the crosstalk between VEGF- and FGF-mediated angiogenic signaling and endothelial sprouting, to gain mechanistic insights and identify novel therapeutic strategies.
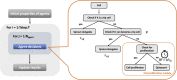
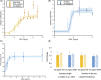
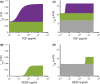
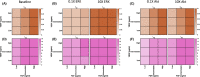
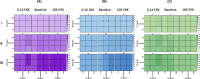
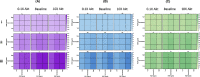
Methods: We constructed an experimentally validated hybrid agent-based mathematical model that characterizes endothelial sprouting driven by FGF- and VEGF-mediated signaling. We predicted the total sprout length, number of sprouts, and average length by the mono- and co-stimulation of FGF and VEGF.
Results: The experimentally fitted and validated model predicts that FGF induces stronger angiogenic responses in the long-term compared with VEGF stimulation. Also, FGF plays a dominant role in the combination effects in endothelial sprouting. Moreover, the model suggests that ERK and Akt pathways and cellular responses contribute differently to the sprouting process. Last, the model predicts that the strategies to modulate endothelial sprouting are context-dependent, and our model can identify potential effective pro- and anti-angiogenic targets under different conditions and study their efficacy.
Conclusions: The model provides detailed mechanistic insight into VEGF and FGF interactions in sprouting angiogenesis. More broadly, this model can be utilized to identify targets that influence angiogenic signaling leading to endothelial sprouting and to study the effects of pro- and anti-angiogenic therapies.
Keywords: agent-based model; angiogenesis; cell signaling; endothelial cell; sprouting.
© 2021 The Authors. Microcirculation published by John Wiley & Sons Ltd.
Figures


Similar articles
-
ERK and Akt exhibit distinct signaling responses following stimulation by pro-angiogenic factors.Cell Commun Signal. 2020 Jul 17;18(1):114. doi: 10.1186/s12964-020-00595-w. Cell Commun Signal. 2020. PMID: 32680529 Free PMC article.
-
Mechanistic insight into activation of MAPK signaling by pro-angiogenic factors.BMC Syst Biol. 2018 Dec 27;12(1):145. doi: 10.1186/s12918-018-0668-5. BMC Syst Biol. 2018. PMID: 30591051 Free PMC article.
-
Endothelial Semaphorin 3fb regulates Vegf pathway-mediated angiogenic sprouting.PLoS Genet. 2021 Aug 23;17(8):e1009769. doi: 10.1371/journal.pgen.1009769. eCollection 2021 Aug. PLoS Genet. 2021. PMID: 34424892 Free PMC article.
-
VEGF and Notch signaling: the yin and yang of angiogenic sprouting.Cell Adh Migr. 2007 Jul-Sep;1(3):133-6. doi: 10.4161/cam.1.3.4978. Epub 2007 Jul 30. Cell Adh Migr. 2007. PMID: 19262131 Free PMC article. Review.
-
Dynamics of endothelial cell behavior in sprouting angiogenesis.Curr Opin Cell Biol. 2010 Oct;22(5):617-25. doi: 10.1016/j.ceb.2010.08.010. Curr Opin Cell Biol. 2010. PMID: 20817428 Review.
Cited by
-
Equine Endothelial Cells Show Pro-Angiogenic Behaviours in Response to Fibroblast Growth Factor 2 but Not Vascular Endothelial Growth Factor A.Int J Mol Sci. 2024 May 30;25(11):6017. doi: 10.3390/ijms25116017. Int J Mol Sci. 2024. PMID: 38892205 Free PMC article.
-
Mechanistic computational modeling of sFLT1 secretion dynamics.bioRxiv [Preprint]. 2025 Feb 18:2025.02.12.637983. doi: 10.1101/2025.02.12.637983. bioRxiv. 2025. Update in: PLoS Comput Biol. 2025 Aug 18;21(8):e1013324. doi: 10.1371/journal.pcbi.1013324. PMID: 40027776 Free PMC article. Updated. Preprint.
-
Mechanistic computational modeling of sFLT1 secretion dynamics.PLoS Comput Biol. 2025 Aug 18;21(8):e1013324. doi: 10.1371/journal.pcbi.1013324. eCollection 2025 Aug. PLoS Comput Biol. 2025. PMID: 40825055 Free PMC article.
-
A perfectly imperfect engine: Utilizing the digital twin paradigm in pulmonary hypertension.Pulm Circ. 2024 Jun 25;14(2):e12392. doi: 10.1002/pul2.12392. eCollection 2024 Apr. Pulm Circ. 2024. PMID: 38933181 Free PMC article. Review.
-
Experiment-based Computational Model Predicts that IL-6 Trans-Signaling Plays a Dominant Role in IL-6 mediated signaling in Endothelial Cells.bioRxiv [Preprint]. 2023 Feb 3:2023.02.03.526721. doi: 10.1101/2023.02.03.526721. bioRxiv. 2023. Update in: NPJ Syst Biol Appl. 2023 Sep 21;9(1):45. doi: 10.1038/s41540-023-00308-2. PMID: 36778489 Free PMC article. Updated. Preprint.
References
-
- Eelen G, Treps L, Li X, Carmeliet P. Basic and therapeutic aspects of angiogenesis updated. Circ Res. 2020;127(2):310‐329. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

