Major tail proteins of bacteriophages of the order Caudovirales
- PMID: 34890646
- PMCID: PMC8718954
- DOI: 10.1016/j.jbc.2021.101472
Major tail proteins of bacteriophages of the order Caudovirales
Abstract
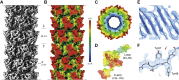
Technological advances in cryo-EM in recent years have given rise to detailed atomic structures of bacteriophage tail tubes-a class of filamentous protein assemblies that could previously only be studied on the atomic scale in either their monomeric form or when packed within a crystal lattice. These hollow elongated protein structures, present in most bacteriophages of the order Caudovirales, connect the DNA-containing capsid with a receptor function at the distal end of the tail and consist of helical and polymerized major tail proteins. However, the resolution of cryo-EM data for these systems differs enormously between different tail tube types, partly inhibiting the building of high-fidelity models and barring a combination with further structural biology methods. Here, we review the structural biology efforts within this field and highlight the role of integrative structural biology approaches that have proved successful for some of these systems. Finally, we summarize the structural elements of major tail proteins and conceptualize how different amounts of tail tube flexibility confer heterogeneity within cryo-EM maps and, thus, limit high-resolution reconstructions.
Keywords: NMR; bacteriophage; cryo-EM; crystallography; phage; phage tail; protein dynamics; solid-state NMR; structural biology; virus.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures





References
-
- Dedrick R.M., Guerrero-Bustamante C.A., Garlena R.A., Russell D.A., Ford K., Harris K., Gilmour K.C., Soothill J., Jacobs-Sera D., Schooley R.T., Hatfull G.F., Spencer H. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019;25:730–733. - PMC - PubMed
-
- Ackermann H.W. 5500 Phages examined in the electron microscope. Arch. Virol. 2007;152:227–243. - PubMed
-
- Adriaenssens E.M., Sullivan M.B., Knezevic P., van Zyl L.J., Sarkar B.L., Dutilh B.E., Alfenas-Zerbini P., Łobocka M., Tong Y., Brister J.R., Moreno Switt A.I., Klumpp J., Aziz R.K., Barylski J., Uchiyama J., et al. Taxonomy of prokaryotic viruses: 2018-2019 update from the ICTV Bacterial and Archaeal Viruses Subcommittee. Arch. Virol. 2020;165:1253–1260. - PubMed
-
- Ackermann H.W. Bacteriophage observations and evolution. Res. Microbiol. 2003;154:245–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

