Neuropeptide Y1 receptor antagonism protects β-cells and improves glycemic control in type 2 diabetes
- PMID: 34890851
- PMCID: PMC8733231
- DOI: 10.1016/j.molmet.2021.101413
Neuropeptide Y1 receptor antagonism protects β-cells and improves glycemic control in type 2 diabetes
Abstract
Objectives: Loss of functional β-cell mass is a key factor contributing to poor glycemic control in advanced type 2 diabetes (T2D). We have previously reported that the inhibition of the neuropeptide Y1 receptor improves the islet transplantation outcome in type 1 diabetes (T1D). The aim of this study was to identify the pathophysiological role of the neuropeptide Y (NPY) system in human T2D and further evaluate the therapeutic potential of using the Y1 receptor antagonist BIBO3304 to improve β-cell function and survival in T2D.
Methods: The gene expression of the NPY system in human islets from nondiabetic subjects and subjects with T2D was determined and correlated with the stimulation index. The glucose-lowering and β-cell-protective effects of BIBO3304, a selective orally bioavailable Y1 receptor antagonist, in high-fat diet (HFD)/multiple low-dose streptozotocin (STZ)-induced and genetically obese (db/db) T2D mouse models were assessed.
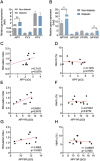
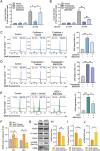
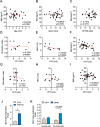
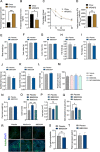
Results: In this study, we identified a more than 2-fold increase in NPY1R and its ligand, NPY mRNA expression in human islets from subjects with T2D, which was significantly associated with reduced insulin secretion. Consistently, the pharmacological inhibition of Y1 receptors by BIBO3304 significantly protected β cells from dysfunction and death under multiple diabetogenic conditions in islets. In a preclinical study, we demonstrated that the inhibition of Y1 receptors by BIBO3304 led to reduced adiposity and enhanced insulin action in the skeletal muscle. Importantly, the Y1 receptor antagonist BIBO3304 treatment also improved β-cell function and preserved functional β-cell mass, thereby resulting in better glycemic control in both HFD/multiple low-dose STZ-induced and db/db T2D mice.
Conclusions: Our results revealed a novel causal link between increased islet NPY-Y1 receptor gene expression and β-cell dysfunction and failure in human T2D, contributing to the understanding of the pathophysiology of T2D. Furthermore, our results demonstrate that the inhibition of the Y1 receptor by BIBO3304 represents a potential β-cell-protective therapy for improving functional β-cell mass and glycemic control in T2D.
Keywords: Insulin secretion; NPY; Type 2 diabetes; Y1 receptor; β-Cell.
Copyright © 2021 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures




References
-
- Wild S., et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. - PubMed
-
- Harding J.L., et al. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62(1):3–16. - PubMed
-
- Weir G.C., Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53(Suppl. 3):S16–S21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

