A poly(ethylene glycol) three-dimensional bone marrow hydrogel
- PMID: 34890973
- PMCID: PMC8890749
- DOI: 10.1016/j.biomaterials.2021.121270
A poly(ethylene glycol) three-dimensional bone marrow hydrogel
Abstract
Three-dimensional (3D) hydrogels made from synthetic polymers have emerged as in vitro cell culture platforms capable of representing the extracellular geometry, modulus, and water content of tissues in a tunable fashion. Hydrogels made from these otherwise non-bioactive polymers can be decorated with short peptides derived from proteins naturally found in tissues to support cell viability and direct phenotype. We identified two key limitations that limit the ability of this class of materials to recapitulate real tissue. First, these environments typically display between 1 and 3 bioactive peptides, which vastly underrepresents the diversity of proteins found in the extracellular matrix (ECM) of real tissues. Second, peptides chosen are ubiquitous in ECM and not derived from proteins found in specific tissues, per se. To overcome this critical limitation in hydrogel design and functionality, we developed an approach to incorporate the complex and specific protein signature of bone marrow into a poly (ethylene glycol) (PEG) hydrogel. This bone marrow hydrogel mimics the elasticity of marrow and has 20 bone marrow-specific and cell-instructive peptides. We propose this tissue-centric approach as the next generation of 3D hydrogel design for applications in tissue engineering and beyond.
Keywords: 3D biomaterial; Mesenchymal stem cell; Peptide; Stiffness; Tissue mimic; integrin.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures

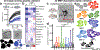
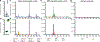

References
-
- Uhlén M. et al. Tissue-based map of the human proteome. Science 347, 1260419 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

