Tumor immune microenvironment lncRNAs
- PMID: 34891154
- PMCID: PMC8769899
- DOI: 10.1093/bib/bbab504
Tumor immune microenvironment lncRNAs
Abstract
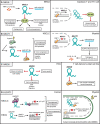
Long non-coding ribonucleic acids (RNAs) (lncRNAs) are key players in tumorigenesis and immune responses. The nature of their cell type-specific gene expression and other functional evidence support the idea that lncRNAs have distinct cellular functions in the tumor immune microenvironment (TIME). To date, the majority of lncRNA studies have heavily relied on bulk RNA-sequencing data in which various cell types contribute to an averaged signal, limiting the discovery of cell type-specific lncRNA functions. Single-cell RNA-sequencing (scRNA-seq) is a potential solution for tackling this limitation despite the lack of annotations for low abundance yet cell type-specific lncRNAs. Hence, updated annotations and further understanding of the cellular expression of lncRNAs will be necessary for characterizing cell type-specific functions of lncRNA genes in the TIME. In this review, we discuss lncRNAs that are specifically expressed in tumor and immune cells, summarize the regulatory functions of the lncRNAs at the cell type level and highlight how a scRNA-seq approach can help to study the cell type-specific functions of TIME lncRNAs.
Keywords: bulk RNA-sequencing; cell type-specific expression; immune cells; long non-coding RNA; single-cell RNA-sequencing; tumor immune microenvironment.
© The Author(s) 2021. Published by Oxford University Press.
Figures






References
-
- Xu J, Bai J, Zhang X, et al. . A comprehensive overview of lncRNA annotation resources. Brief Bioinform 2017;18(2):236–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

