COVID-19 mRNA vaccine may trigger subacute thyroiditis
- PMID: 34893014
- PMCID: PMC8904015
- DOI: 10.1080/21645515.2021.2013083
COVID-19 mRNA vaccine may trigger subacute thyroiditis
Abstract
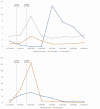
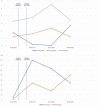
Subacute thyroiditis is the most common cause of painful thyroiditis, which usually occurs after an acute viral upper respiratory tract infection. Rare cases of subacute thyroiditis have been reported after administration of viral vaccines. Here, we report four cases of subacute thyroiditis after administration of the COVID-19 mRNA vaccine (Pfizer/BioNTech®). We describe the clinical, laboratory and imaging features of five cases of subacute thyroiditis after COVID-19 mRNA vaccine (Pfizer/BioNTech®). COVID-19 mRNA vaccine (Pfizer/BioNTech®)-associated subacute thyroiditis may present with clinical findings typical of classic subacute thyroiditis such as fever, neck pain, weakness, and tremor within a few days following vaccination. Subacute thyroiditis may be focal or may progress with diffuse bilateral involvement. Depending on the extent of subacute thyroiditis involvement, significant increases in acute-phase reactants can be observed. COVID-19 mRNA vaccine (Pfizer/BioNTech®) associated subacute thyroiditis responds quite well to non-steroidal anti-inflammatory therapy. Clinicians should be aware of the risk of developing subacute thyroiditis after vaccination.
Keywords: COVID-19; SARS-CoV-2 vaccine; subacute thyroiditis.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures

References
-
- Certain medical conditions and risk for severe COVID-19 illness | CDC [Internet]. [accessed 2020. Sep 7]. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-....
-
- Lisco G, De Tullio A, Stragapede A, Solimando AG, Albanese F, Capobianco M, Giagulli VA, Guastamacchia E, De Pergola G, Vacca A, et al. COVID-19 and the endocrine system: a comprehensive review on the theme. J Clin Med [Internet]. 2021. [accessed 2021 Aug 17];10:2920. doi:10.3390/jcm10132920. - DOI - PMC - PubMed
-
- Ku CR, Jung KY, Ahn CH, Moon JS, Lee JH, Kim EH, Kwon H, Kim HK, Suh S, Hong S, et al. COVID-19 vaccination for endocrine patients: a position statement from the Korean Endocrine Society. Endocrinol Metab [Internet]. 2021. [accessed 2021 Aug 17];36:757–65. doi:10.3803/EnM.2021.404. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
