The IRF2/CENP-N/AKT signaling axis promotes proliferation, cell cycling and apoptosis resistance in nasopharyngeal carcinoma cells by increasing aerobic glycolysis
- PMID: 34893086
- PMCID: PMC8662847
- DOI: 10.1186/s13046-021-02191-3
The IRF2/CENP-N/AKT signaling axis promotes proliferation, cell cycling and apoptosis resistance in nasopharyngeal carcinoma cells by increasing aerobic glycolysis
Abstract
Background: Centromere protein N (CENP-N) has been reported to be highly expressed in malignancies, but its role and mechanism in nasopharyngeal carcinoma (NPC) are unknown.
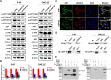
Methods: Abnormal CENP-N expression from NPC microarrays of GEO database was analyzed. CENP-N expression level was confirmed in NPC tissues and cell lines. Stable CENP-N knockdown and overexpression NPC cell lines were established, and transcriptome sequencing after CENP-N knockdown was performed. In vitro and in vivo experiments were performed to test the impact of CENP-N knockdown in NPC cells. ChIP and dual luciferase reporter assays were used to verify the combination of IRF2 and CENP-N. Western blot analysis, cellular immunofluorescence, immunoprecipitation and GST pulldown assays were used to verify the combination of CENP-N and AKT.
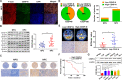
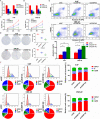
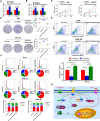
Results: CENP-N was confirmed to be aberrantly highly expressed in NPC tissues and cell lines and to be associated with high 18F-FDG uptake in cancer nests and poor patient prognosis. Transcriptome sequencing after CENP-N knockdown revealed that genes with altered expression were enriched in pathways related to glucose metabolism, cell cycle regulation. CENP-N knockdown inhibited glucose metabolism, cell proliferation, cell cycling and promoted apoptosis. IRF2 is a transcription factor for CENP-N and directly promotes CENP-N expression in NPC cells. CENP-N affects the glucose metabolism, proliferation, cell cycling and apoptosis of NPC cells in vitro and in vivo through the AKT pathway. CENP-N formed a complex with AKT in NPC cells. Both an AKT inhibitor (MK-2206) and a LDHA inhibitor (GSK2837808A) blocked the effect of CENP-N overexpression on NPC cells by promoting aerobic glycolysis, proliferation, cell cycling and apoptosis resistance.
Conclusions: The IRF2/CENP-N/AKT axis promotes malignant biological behaviors in NPC cells by increasing aerobic glycolysis, and the IRF2/CENP-N/AKT signaling axis is expected to be a new target for NPC therapy.
Keywords: AKT; Aerobic glycolysis; CENP-N; Gglucose metabolism; IRF2; NPC.
© 2021. The Author(s).
Conflict of interest statement
None.
Figures




References
-
- Tang LL, Chen WQ, Xue WQ, He YQ, Zheng RS, Zeng YX, et al. Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer Lett. 2016;374(1):22–30. - PubMed
-
- Carioli G, Negri E, Kawakita D, Garavello W, La Vecchia C, Malvezzi M. Global trends in nasopharyngeal cancer mortality since 1970 and predictions for 2020: focus on low-risk areas. Int J Cancer. 2017;140(10):2256–2264. - PubMed
-
- Chen YP, Ismaila N, Chua MLK, Colevas AD, Haddad R, Huang SH, et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol. 2021;39(7):840–859. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

