Humoral anti-SARS-CoV-2 immune response after two doses of Comirnaty vaccine in nursing home residents by previous infection status
- PMID: 34893341
- PMCID: PMC8639399
- DOI: 10.1016/j.vaccine.2021.11.086
Humoral anti-SARS-CoV-2 immune response after two doses of Comirnaty vaccine in nursing home residents by previous infection status
Abstract
Background: Coronavirus disease 2019 (COVID-19) is associated with increased morbidity and mortality in older adults. Although the advent of the first vaccines has significantly reduced these rates, data on older adults in clinical trials are scarce.
Objectives: We quantified and compared the humoral response in individuals with vs. without pre-existing seropositivity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in a cohort of 69 patients living in a nursing home and who had received the recommended two doses of the Comirnaty (Pfizer-BioNTech®) vaccine.
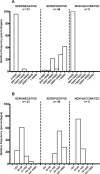
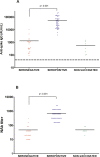
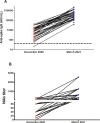
Results: All 69 patients (100%) tested positive for antibodies against SARS-CoV-2 at 2 months post-vaccination. Residents with a pre-vaccination infection had significantly higher titers of anti-spike 1 IgG than those with no prior infection (median [interquartile range]: 55,726 [14463-78852] vs. 1314 [272-1249] arbitrary units, respectively; p < 0.001). The same result was observed for neutralizing antibodies titers (704 [320-1280] vs. 47 [20-40] respectively; p < 0.001). Between the pre-vaccination and post-vaccination periods, for IgG and neutralizing antibodies, we observed a 49 and 8-fold increase respectively. In comparison to the wild-type Receptor Binding Domain (RBD), the binding capacity of these vaccine sera was significantly decreased on the B.1.351 and P.1 variants RBD but not decreased with respect to the B.1.1.7 RBD. Although all nursing home residents developed a humoral response following Comirnaty vaccine, its intensity appeared to depend on the pre-vaccination serological status.
Conclusion: Our results raise the question of how many doses of vaccine should be administered in older and how long the protection will be effective.
Keywords: COVID-19; Humoral vaccine response; SARS-CoV-2; Serological assay.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

