Biased M1 muscarinic receptor mutant mice show accelerated progression of prion neurodegenerative disease
- PMID: 34893539
- PMCID: PMC8685681
- DOI: 10.1073/pnas.2107389118
Biased M1 muscarinic receptor mutant mice show accelerated progression of prion neurodegenerative disease
Abstract
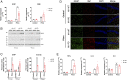
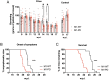
There are currently no treatments that can slow the progression of neurodegenerative diseases, such as Alzheimer's disease (AD). There is, however, a growing body of evidence that activation of the M1 muscarinic acetylcholine receptor (M1-receptor) can not only restore memory loss in AD patients but in preclinical animal models can also slow neurodegenerative disease progression. The generation of an effective medicine targeting the M1-receptor has however been severely hampered by associated cholinergic adverse responses. By using genetically engineered mouse models that express a G protein-biased M1-receptor, we recently established that M1-receptor mediated adverse responses can be minimized by ensuring activating ligands maintain receptor phosphorylation/arrestin-dependent signaling. Here, we use these same genetic models in concert with murine prion disease, a terminal neurodegenerative disease showing key hallmarks of AD, to establish that phosphorylation/arrestin-dependent signaling delivers neuroprotection that both extends normal animal behavior and prolongs the life span of prion-diseased mice. Our data point to an important neuroprotective property inherent to the M1-receptor and indicate that next generation M1-receptor ligands designed to drive receptor phosphorylation/arrestin-dependent signaling would potentially show low adverse responses while delivering neuroprotection that will slow disease progression.
Keywords: GPCR; M1 muscarinic acetylcholine receptor; neurodegenerative disease; phosphorylation.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Prince M., et al. , “World Alzheimer Report 2015: The global impact of dementia” (Alzheimer’s Disease International, London, 2015).
-
- Courtney C., et al. ; AD2000 Collaborative Group, Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): Randomised double-blind trial. Lancet 363, 2105–2115 (2004). - PubMed
-
- Inglis F., The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int. J. Clin. Pract. Suppl. 127, 45–63 (2002). - PubMed
-
- Svensson A.-L., Alafuzoff I., Nordberg A., Characterization of muscarinic receptor subtypes in Alzheimer and control brain cortices by selective muscarinic antagonists. Brain Res. 596, 142–148 (1992). - PubMed
-
- Levey A. I., Immunological localization of m1-m5 muscarinic acetylcholine receptors in peripheral tissues and brain. Life Sci. 52, 441–448 (1993). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

