Endothelial dysfunction contributes to severe COVID-19 in combination with dysregulated lymphocyte responses and cytokine networks
- PMID: 34893580
- PMCID: PMC8661333
- DOI: 10.1038/s41392-021-00819-6
Endothelial dysfunction contributes to severe COVID-19 in combination with dysregulated lymphocyte responses and cytokine networks
Abstract
The systemic processes involved in the manifestation of life-threatening COVID-19 and in disease recovery are still incompletely understood, despite investigations focusing on the dysregulation of immune responses after SARS-CoV-2 infection. To define hallmarks of severe COVID-19 in acute disease (n = 58) and in disease recovery in convalescent patients (n = 28) from Hannover Medical School, we used flow cytometry and proteomics data with unsupervised clustering analyses. In our observational study, we combined analyses of immune cells and cytokine/chemokine networks with endothelial activation and injury. ICU patients displayed an altered immune signature with prolonged lymphopenia but the expansion of granulocytes and plasmablasts along with activated and terminally differentiated T and NK cells and high levels of SARS-CoV-2-specific antibodies. The core signature of seven plasma proteins revealed a highly inflammatory microenvironment in addition to endothelial injury in severe COVID-19. Changes within this signature were associated with either disease progression or recovery. In summary, our data suggest that besides a strong inflammatory response, severe COVID-19 is driven by endothelial activation and barrier disruption, whereby recovery depends on the regeneration of the endothelial integrity.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




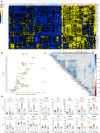

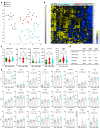
References
-
- Thi Nhu Thao T, et al. Rapid reconstruction of SARS-CoV-2 using a synthetic genomics platform. Nature. 2020;582:561–565. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DFG FA-483/1-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- DFG FA-483/1-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- DZIF TTU-IICH 07_913/Deutsches Zentrum für Infektionsforschung (German Center for Infection Research)
- DZIF TTU-IICH 07_913/Deutsches Zentrum für Infektionsforschung (German Center for Infection Research)
- ImProVIT/Niedersächsische Ministerium für Wissenschaft und Kultur (Lower Saxony Ministry of Science and Culture)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

