Rational design and construction of multi-copy biomanufacturing islands in mammalian cells
- PMID: 34893882
- PMCID: PMC8754653
- DOI: 10.1093/nar/gkab1214
Rational design and construction of multi-copy biomanufacturing islands in mammalian cells
Abstract
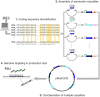
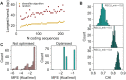
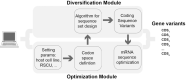
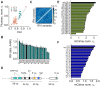
Cell line development is a critical step in the establishment of a biopharmaceutical manufacturing process. Current protocols rely on random transgene integration and amplification. Due to considerable variability in transgene integration profiles, this workflow results in laborious screening campaigns before stable producers can be identified. Alternative approaches for transgene dosage increase and integration are therefore highly desirable. In this study, we present a novel strategy for the rapid design, construction, and genomic integration of engineered multiple-copy gene constructs consisting of up to 10 gene expression cassettes. Key to this strategy is the diversification, at the sequence level, of the individual gene cassettes without altering their protein products. We show a computational workflow for coding and regulatory sequence diversification and optimization followed by experimental assembly of up to nine gene copies and a sentinel reporter on a contiguous scaffold. Transient transfections in CHO cells indicates that protein expression increases with the gene copy number on the scaffold. Further, we stably integrate these cassettes into a pre-validated genomic locus. Altogether, our findings point to the feasibility of engineering a fully mapped multi-copy recombinant protein 'production island' in a mammalian cell line with greatly reduced screening effort, improved stability, and predictable product titers.
© The Author(s) 2021. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



References
-
- Gronemeyer P., Ditz R., Strube J.. Trends in upstream and downstream process development for antibody manufacturing. Bioengineering. 2014; 1:188–212. - PubMed
-
- Walsh G. Biopharmaceutical benchmarks 2018. Nat. Biotechnol. 2018; 36:1136–1145. - PubMed
-
- Wurm F.M. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat. Biotechnol. 2004; 22:1393–1398. - PubMed
-
- Fan L., Kadura I., Krebs L.E., Hatfield C.C., Shaw M.M., Frye C.C.. Improving the efficiency of CHO cell line generation using glutamine synthetase gene knockout cells. Biotechnol. Bioeng. 2012; 109:1007–1015. - PubMed
-
- Cacciatore J.J., Chasin L.A., Leonard E.F.. Gene amplification and vector engineering to achieve rapid and high-level therapeutic protein production using the Dhfr-based CHO cell selection system. Biotechnol. Adv. 2010; 28:673–681. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

