Hierarchically Multivalent Peptide-Nanoparticle Architectures: A Systematic Approach to Engineer Surface Adhesion
- PMID: 34894089
- PMCID: PMC8811846
- DOI: 10.1002/advs.202103098
Hierarchically Multivalent Peptide-Nanoparticle Architectures: A Systematic Approach to Engineer Surface Adhesion
Abstract
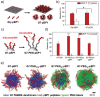
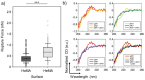
The multivalent binding effect has been the subject of extensive studies to modulate adhesion behaviors of various biological and engineered systems. However, precise control over the strong avidity-based binding remains a significant challenge. Here, a set of engineering strategies are developed and tested to systematically enhance the multivalent binding of peptides in a stepwise manner. Poly(amidoamine) (PAMAM) dendrimers are employed to increase local peptide densities on a substrate, resulting in hierarchically multivalent architectures (HMAs) that display multivalent dendrimer-peptide conjugates (DPCs) with various configurations. To control binding behaviors, effects of the three major components of the HMAs are investigated: i) poly(ethylene glycol) (PEG) linkers as spacers between conjugated peptides; ii) multiple peptides on the DPCs; and iii) various surface arrangements of HMAs (i.e., a mixture of DPCs each containing different peptides vs DPCs cofunctionalized with multiple peptides). The optimized HMA configuration enables significantly enhanced target cell binding with high selectivity compared to the control surfaces directly conjugated with peptides. The engineering approaches presented herein can be applied individually or in combination, providing guidelines for the effective utilization of biomolecular multivalent interactions using DPC-based HMAs.
Keywords: binding avidity; dendrimer-peptide conjugate; hierarchically multivalent architectures; multivalent binding; peptide engineering.
© 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- a) Jiménez Blanco J. L., Ortiz Mellet C., García Fernández J. M., Chem. Soc. Rev. 2013, 42, 4518; - PubMed
- b) Worstell N. C., Singla A., Saenkham P., Galbadage T., Sule P., Lee D., Mohr A., Kwon J. S., Cirillo J. D., Wu H. J., Sci. Rep. 2018, 8, 8419; - PMC - PubMed
- c) McKenzie M., Ha S. M., Rammohan A., Radhakrishnan R., Ramakrishnan N., Biophys. J. 2018, 114, 1830. - PMC - PubMed
-
- Zhang K., Gao H., Deng R., Li J., Angew. Chem., Int. Ed. 2019, 58, 4790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
