Aiming for Functional Cure With Established and Novel Therapies for Chronic Hepatitis B
- PMID: 34894108
- PMCID: PMC9035586
- DOI: 10.1002/hep4.1875
Aiming for Functional Cure With Established and Novel Therapies for Chronic Hepatitis B
Abstract
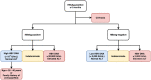
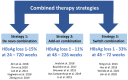
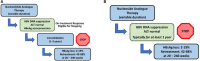
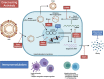
Chronic hepatitis B virus (HBV) infection remains difficult to cure due to the persistent, self-replenishing nature of the viral genome and impaired host immune responses. Current treatment goals for chronic hepatitis B (CHB) are to prevent or significantly delay liver-related adverse outcomes and death, and two types of treatments are available: nucleos(t)ide analogues (NAs) and interferons (IFNs). NAs effectively suppress HBV replication, and IFNs improve serological response rates, thereby decreasing the risk of adverse outcomes. However, their efficacy in attaining serological responses, especially functional cure (i.e., loss of serum hepatitis B surface antigen), is very limited. Various strategies such as stopping antiviral therapy or combining therapies have been investigated to enhance response, but efficacy is only modestly improved. Importantly, the development of novel direct-acting antivirals and immunomodulators is underway to improve treatment efficacy and enhance rates of functional cure. The present review provides an overview of the treatment goals and indications, the possibility of expanding indications, and the safety and efficacy of different treatment strategies involving established and/or novel therapies as we continue our search for a cure.
© 2021 The Authors. Hepatology Communications published by Wiley Periodicals LLC on behalf of American Association for the Study of Liver Diseases.
Figures
References
-
- World Health Organization . Global Hepatitis Report. 2017. https://www.who.int/publications/i/item/global‐hepatitis‐report‐2017. Accessed June 30, 2021.
-
- Anderson RT, Choi HSJ, Lenz O, Peters MG, Janssen HLA, Mishra P, et al. Association between seroclearance of hepatitis B surface antigen and long‐term clinical outcomes of patients with chronic hepatitis B virus infection: systematic review and meta‐analysis. Clin Gastroenterol Hepatol 2021;19:463‐472. - PubMed
-
- Lampertico P, Agarwal K, Berg T, Buti M, Janssen HLA, Papatheodoridis G, et al. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370‐398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

