The Anti-Circumsporozoite Antibody Response of Children to Seasonal Vaccination With the RTS,S/AS01E Malaria Vaccine
- PMID: 34894221
- PMCID: PMC9464075
- DOI: 10.1093/cid/ciab1017
The Anti-Circumsporozoite Antibody Response of Children to Seasonal Vaccination With the RTS,S/AS01E Malaria Vaccine
Abstract
Background: A trial in African children showed that combining seasonal vaccination with the RTS,S/AS01E vaccine with seasonal malaria chemoprevention reduced the incidence of uncomplicated and severe malaria compared with either intervention given alone. Here, we report on the anti-circumsporozoite antibody response to seasonal RTS,S/AS01E vaccination in children in this trial.
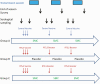
Methods: Sera from a randomly selected subset of children collected before and 1 month after 3 priming doses of RTS,S/AS01E and before and 1 month after 2 seasonal booster doses were tested for anti-circumsporozoite antibodies using enzyme-linked immunosorbent assay. The association between post-vaccination antibody titer and incidence of malaria was explored.
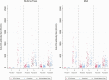
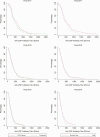
Results: A strong anti-circumsporozoite antibody response to 3 priming doses of RTS,S/AS01E was seen (geometric mean titer, 368.9 enzyme-linked immunosorbent assay units/mL), but titers fell prior to the first booster dose. A strong antibody response to an annual, pre-malaria transmission season booster dose was observed, but this was lower than after the primary vaccination series and lower after the second than after the first booster dose (ratio of geometric mean rise, 0.66; 95% confidence interval [CI], .57-.77). Children whose antibody response was in the upper tercile post-vaccination had a lower incidence of malaria during the following year than children in the lowest tercile (hazard ratio, 0.43; 95% CI, .28-.66).
Conclusions: Seasonal vaccination with RTS,S/AS01E induced a strong booster antibody response that was lower after the second than after the first booster dose. The diminished antibody response to the second booster dose was not associated with diminished efficacy.
Clinical trials registration: NCT03143218.
Keywords: Burkina Faso; Mali; RTS; S/AS01E vaccine; anti-circumsporozoite antibody; seasonal vaccination.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. O. O.-A. is an employee of the GSK group of companies and has restricted shares in the GSK group of companies. A. D. reports a research grant from Grand Challenges Canada to test safety and efficacy of the PfSPZ vaccine for pregnant women and unborn children and a research contract with Oxford University to conduct a phase 3 randomized, controlled multicenter trial to evaluate the efficacy of the R21/Matrix-M vaccine in African children against clinical malaria outside of the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- World Health Organization. High burden to high impact. A targeted malaria response. WHO/CDS/GMP/2018.25.Rev 1. Geneva, Switzerland: World Health Organization, 2019.
-
- Chandramohan D, Dicko A, Zongo I, et al. . Effect of adding azithromycin to seasonal malaria chemoprevention. N Engl J Med 2019; 380:2197–206. - PubMed

