Processing Adipose Tissue to Make it More Stable When Used for Refilling: A Morphologic and Immunohistochemistry Evaluation
- PMID: 34894844
- PMCID: PMC8679401
- DOI: 10.1177/00469580211061030
Processing Adipose Tissue to Make it More Stable When Used for Refilling: A Morphologic and Immunohistochemistry Evaluation
Abstract
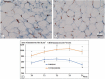
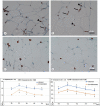
Breast reconstruction has gained from lipofilling the possibility to recover the aesthetic outcome of anatomical profile in a more natural appearance. However, until today, the long-term graft survival remains unpredictable, and sometimes it does not guarantee a well-adequate aesthetic result. In the present work, the morphological changes, occurring in fat mass used for refilling, harvested by the Coleman's procedure or through the washing/fragmenting procedure were analysed. Adipocyte size; immunohistochemistry against CD8, CD31, CD68 and M2-type macrophages and catalase enzyme, were analysed in vitro on fat mass cultured for 4 weeks. Our observation reveals an increase of connective tissue around the mass and a high number of immune cells occurrence in fat mass harvested by the Coleman's procedure. Instead, the washing/fragmented procedure would reduce the number of immune cells within the fat mass, increase the size of adipocytes, and cause a wider presence of active vessels profile and greater catalase expression. We hypothesize that the fat mass processed by the Coleman's procedure would remain more reactive due to a higher number of immune and macrophages cells, prone to develop cystic formation, leading to a suboptimal integration in the recipient site. On the other hand, the conditions more prone to realize an optimal integration would occur in the fat mass processed by the washing/fragmenting procedure: a reduced number of immune cells, low amount of connective tissue, presence of larger adipocytes. Follow-up monitoring did support our conclusion, as we observed a reduction of re-intervention for refilling procedure in patients treated with the washing/fragmenting procedure.
Keywords: M2 macrophages; adipose cell; breast refilling; fat transplantation; lipofilling.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

