Synthesis and biological evaluation of halogenated phenoxychalcones and their corresponding pyrazolines as cytotoxic agents in human breast cancer
- PMID: 34894967
- PMCID: PMC8667918
- DOI: 10.1080/14756366.2021.1998023
Synthesis and biological evaluation of halogenated phenoxychalcones and their corresponding pyrazolines as cytotoxic agents in human breast cancer
Abstract
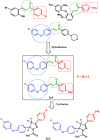
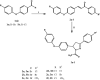
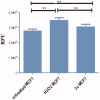
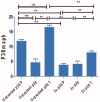
Novel halogenated phenoxychalcones 2a-f and their corresponding N-acetylpyrazolines 3a-f were synthesised and evaluated for their anticancer activities against breast cancer cell line (MCF-7) and normal breast cell line (MCF-10a), compared with staurosporine. All compounds showed moderate to good cytotoxic activity when compared to control. Compound 2c was the most active, with IC50 = 1.52 µM and selectivity index = 15.24. Also, chalcone 2f showed significant cytotoxic activity with IC50 = 1.87 µM and selectivity index = 11.03. Compound 2c decreased both total mitogen activated protein kinase (p38α MAPK) and phosphorylated enzyme in MCF-7 cells, suggesting its ability to decrease cell proliferation and survival. It also showed the ability to induce ROS in MCF-7 treated cells. Compound 2c exhibited apoptotic behaviour in MCF-7 cells due to cell accumulation in G2/M phase and elevation in late apoptosis 57.78-fold more than control. Docking studies showed that compounds 2c and 2f interact with p38alpha MAPK active sites.
Keywords: Halogenated chalcones; ROS; breast cancer; cell cycle profile; diaryl ether; p38 MAPK; pyrazoline.
Conflict of interest statement
The authors declare that they have no conflicts of interest. The authors alone are responsible for the content and writing of this manuscript.
Figures








References
-
- Boström J, Brown DG, Young RJ, Keserü GM.. Expanding the medicinal chemistry synthetic toolbox. Nat Rev Drug Discov 2018;17:709–27. - PubMed
-
- Singh I, Rani R, Luxami V, Paul K.. Synthesis of 5-(4-(1H-phenanthro[9,10-d]imidazol-2-yl)benzylidene)thiazolidine-2,4-dione as promising DNA and serum albumin-binding agents and evaluation of antitumor activity. Eur J Med Chem 2019;166:267–80. - PubMed
-
- El-dash Y, Elzayat E, Abdou AM, Hassan RA.. Novel thienopyrimidine-aminothiazole hybrids: design, synthesis, antimicrobial screening, anticancer activity, effects on cell cycle profile, caspase-3 mediated apoptosis and VEGFR-2 inhibition. Bioorganic Chemistry 2021;114:105137. - PubMed
-
- Caruso E, Malacarne MC, Marras E, et al. New BODIPYs for photodynamic therapy (PDT): synthesis and activity on human cancer cell lines. Bioorg Med Chem 2020;28:115737. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
