MiR-26b-5p inhibits cell proliferation and EMT by targeting MYCBP in triple-negative breast cancer
- PMID: 34895159
- PMCID: PMC8903572
- DOI: 10.1186/s11658-021-00288-3
MiR-26b-5p inhibits cell proliferation and EMT by targeting MYCBP in triple-negative breast cancer
Abstract
Background: The study was designed to elucidate the association and functional roles of miR-26b-5p and c-MYC binding protein (MYCBP) in triple-negative breast cancer (TNBC).
Method: Luciferase reporter assay was used to confirm the relationship between miR-26b-5p and MYCBP in TNBC cells. The expression levels of miR-26b-5p and MYCBP in tissue specimens and cell lines were determined using reverse transcription-quantitative PCR. Cell proliferation, migration and invasion were assessed using CCK-8 assay, colony formation and transwell assay.
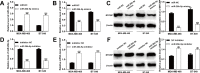
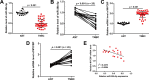
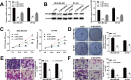
Results: We first observed that miR-26b-5p directly targets the 3'-UTR of MYCBP to inhibit MYCBP expression in MDA-MB-468 and BT-549 cells. The expression of miR-26b-5p was inversely correlated with MYCBP expression in TNBC tissues. We further demonstrated that MYCBP knockdown suppressed the proliferation, migration and invasion of TNBC cells. Furthermore, MYCBP overexpression counteracted the suppressive effect of miR-26b-5p on TNBC cell behaviors. Western blot analysis demonstrated that the E-cadherin protein level was increased, while protein levels of N-cadherin and vimentin were decreased in cells transfected with miR-26b-5p, which were all reversed by ectopic expression of MYCBP.
Conclusions: In summary, our findings revealed the tumor suppressive role of miR-26b-5p in regulating TNBC cell proliferation and mobility, possibly by targeting MYCBP.
Keywords: EMT; MYCBP; Triple-negative breast cancer; miR-26b-5p.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

