MRI for Rectal Cancer: Staging, mrCRM, EMVI, Lymph Node Staging and Post-Treatment Response
- PMID: 34895835
- PMCID: PMC8966586
- DOI: 10.1016/j.clcc.2021.10.007
MRI for Rectal Cancer: Staging, mrCRM, EMVI, Lymph Node Staging and Post-Treatment Response
Abstract
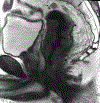
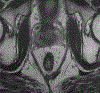
Rectal cancer is a relatively common malignancy in the United States. Magnetic resonance imaging (MRI) of rectal cancer has evolved tremendously in recent years, and has become a key component of baseline staging and treatment planning. In addition to assessing the primary tumor and locoregional lymph nodes, rectal MRI can be used to help with risk stratification by identifying high-risk features such as extramural vascular invasion and can assess treatment response for patients receiving neoadjuvant therapy. As the practice of rectal MRI continues to expand further into academic centers and private practices, standard MRI protocols, and reporting are critical. In addition, it is imperative that the radiologists reading these cases work closely with surgeons, medical oncologists, radiation oncologists, and pathologists to ensure we are providing the best possible care to patients. This review aims to provide a broad overview of the role of MRI for rectal cancer.
Keywords: Cancer staging; MRI; Radiology; Rectal cancer.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosure The authors have stated that they have no conflicts of interest.
Figures














References
-
- Al-Sukhni E, Milot L, Fruitman M, Beyene J, Victor JC, Schmocker S, Brown G, McLeod R, Kennedy E. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19(7):2212–23. - PubMed
-
- Costa-Silva L, Brown G. Magnetic resonance imaging of rectal cancer. Magn Reson Imaging Clin N Am. 2013;21(2):385–408. - PubMed
-
- Furey E, Jhaveri KS. Magnetic resonance imaging in rectal cancer. Magn Reson Imaging Clin N Am. 2014;22(2):165–90, v-vi. - PubMed
-
- Beets-Tan RGH, Lambregts DMJ, Maas M, Bipat S, Barbaro B, Curvo-Semedo L, Fenlon HM, Gollub MJ, Gourtsoyianni S, Halligan S, Hoeffel C, Kim SH, Laghi A, Maier A, Rafaelsen SR, Stoker J, Taylor SA, Torkzad MR, Blomqvist L. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28(4):1465–75. - PMC - PubMed
-
- Taylor FG, Swift RI, Blomqvist L, Brown G. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. AJR Am J Roentgenol. 2008;191(6):1827–35. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

