Age dependence of retinal vascular plexus attenuation in the triple transgenic mouse model of Alzheimer's disease
- PMID: 34896306
- PMCID: PMC10155044
- DOI: 10.1016/j.exer.2021.108879
Age dependence of retinal vascular plexus attenuation in the triple transgenic mouse model of Alzheimer's disease
Abstract
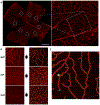
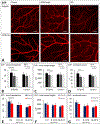
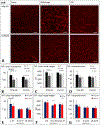
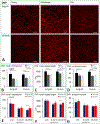
The influence of Alzheimer's disease (AD) progression and severity on the structural and functional integrity of the cerebral vasculature is well recognized. The retina is an extension of the brain; thus, changes in retinal vascular features may serve as markers of AD cerebrovascular pathologies. However, differentiating normal aging-versus AD-induced retinal vascular changes is unresolved. Therefore, we compared and quantified changes in superficial (SVP), intermediate (IVP), and deep (DVP) retinal vascular plexuses in young, middle-age, and old triple transgenic mouse model of AD (3xT-AD) to the changes that occur in age-matched controls (C57BL/6j). We used immunostaining combined with a novel tissue optical clearing approach along with a computational tool for quantitative analysis of vascular network alterations (vessel length and density) in SVP, IVP, and DVP. All three layers had comparable structural features and densities in young 3xTg-AD and control animals. In controls, IVP and DVP densities decreased with aging (-14% to -32% change from young to old, p < 0.05), while no changes were observed in SVP. In contrast, vascular parameters in the transgenic group decreased in all three layers with aging (-12% to -49% change from young to old, p < 0.05). Furthermore, in the old group, SVP and DVP vascular parameters were lower in the transgenics compared to age-matched controls (p < 0.05). Our analysis demonstrates that normal aging and progression of AD lead to various degrees of vascular alterations in the retina. Specifically, compared to normal aging, changes in vascular features of SVP and DVP regions of the retina are accelerated during AD progression. Considering recent advances in the field of depth-resolved imaging of retinal capillary network and microangiography, noninvasive quantitative monitoring of changes in retinal vascular network parameters of SVP and DVP may serve as markers for diagnosis and staging of Alzheimer's disease and discriminating AD-induced vascular attenuation from age-related vasculopathy.
Keywords: 3xTg-AD; Alzheimer's disease; Deep vascular plexus; Intermediate vascular plexus; Retina; Retinal vascular density; Superficial vascular plexus.
Copyright © 2021. Published by Elsevier Ltd.
Figures

Similar articles
-
Quantitative assessment of retinal thickness and vessel density using optical coherence tomography angiography in patients with Alzheimer's disease and glaucoma.PLoS One. 2021 Mar 19;16(3):e0248284. doi: 10.1371/journal.pone.0248284. eCollection 2021. PLoS One. 2021. PMID: 33739997 Free PMC article.
-
Angiography reveals novel features of the retinal vasculature in healthy and diabetic mice.Exp Eye Res. 2015 Sep;138:6-21. doi: 10.1016/j.exer.2015.06.023. Epub 2015 Jun 26. Exp Eye Res. 2015. PMID: 26122048
-
Retinal macroglia changes in a triple transgenic mouse model of Alzheimer's disease.Exp Eye Res. 2014 Oct;127:252-60. doi: 10.1016/j.exer.2014.08.006. Epub 2014 Aug 19. Exp Eye Res. 2014. PMID: 25149907 Free PMC article.
-
Ocular indicators of Alzheimer's: exploring disease in the retina.Acta Neuropathol. 2016 Dec;132(6):767-787. doi: 10.1007/s00401-016-1613-6. Epub 2016 Sep 19. Acta Neuropathol. 2016. PMID: 27645291 Free PMC article. Review.
-
Retinal Changes in Transgenic Mouse Models of Alzheimer's Disease.Curr Alzheimer Res. 2021;18(2):89-102. doi: 10.2174/1567205018666210414113634. Curr Alzheimer Res. 2021. PMID: 33855942 Review.
Cited by
-
Retinal manifestations and their diagnostic significance in Alzheimer's disease.J Alzheimers Dis Rep. 2025 Aug 10;9:25424823251361937. doi: 10.1177/25424823251361937. eCollection 2025 Jan-Dec. J Alzheimers Dis Rep. 2025. PMID: 40799319 Free PMC article. Review.
-
Alzheimer's disease pathophysiology in the Retina.Prog Retin Eye Res. 2024 Jul;101:101273. doi: 10.1016/j.preteyeres.2024.101273. Epub 2024 May 15. Prog Retin Eye Res. 2024. PMID: 38759947 Free PMC article. Review.
-
Lipid metabolism disorder promoting retinal structural and functional damage in ApoE-/- mice with age superposition.Acta Neuropathol Commun. 2025 Jun 4;13(1):125. doi: 10.1186/s40478-025-02043-7. Acta Neuropathol Commun. 2025. PMID: 40468378 Free PMC article.
-
Characterization of the Retinal Circulation of the Mouse.Invest Ophthalmol Vis Sci. 2024 Dec 2;65(14):3. doi: 10.1167/iovs.65.14.3. Invest Ophthalmol Vis Sci. 2024. PMID: 39620830 Free PMC article.
-
The effects of time restricted feeding on age-related changes in the mouse retina.Exp Gerontol. 2024 Sep;194:112510. doi: 10.1016/j.exger.2024.112510. Epub 2024 Jul 5. Exp Gerontol. 2024. PMID: 38964431 Free PMC article.
References
-
- Bennett RE, Robbins AB, Hu M, Cao X, Betensky RA, Clark T, Das S, Hyman BT, 2018. Tau induces blood vessel abnormalities and angiogenesis-related gene expression in P301L transgenic mice and human Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A 115, E1289–E1298. 10.1073/pnas.1710329115. - DOI - PMC - PubMed
-
- Binet F, Cagnone G, Crespo-Garcia S, Hata M, Neault M, Dejda A, Wilson AM, Buscarlet M, Mawambo GT, Howard JP, Diaz-Marin R, Parinot C, Guber V, Pilon F, Juneau R, Laflamme R, Sawchyn C, Boulay K, Leclerc S, Abu-Thuraia A, Côté JF, Andelfinger G, Rezende FA, Sennlaub F, Joyal JS, Mallette FA, Sapieha P, 2020. Neutrophil extracellular traps target senescent vasculature for tissue remodeling in retinopathy. Science 369. 10.1126/SCIENCE.AAY5356. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

