T cell receptor (TCR) signaling in health and disease
- PMID: 34897277
- PMCID: PMC8666445
- DOI: 10.1038/s41392-021-00823-w
T cell receptor (TCR) signaling in health and disease
Abstract
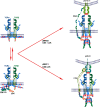
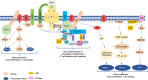
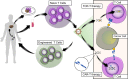
Interaction of the T cell receptor (TCR) with an MHC-antigenic peptide complex results in changes at the molecular and cellular levels in T cells. The outside environmental cues are translated into various signal transduction pathways within the cell, which mediate the activation of various genes with the help of specific transcription factors. These signaling networks propagate with the help of various effector enzymes, such as kinases, phosphatases, and phospholipases. Integration of these disparate signal transduction pathways is done with the help of adaptor proteins that are non-enzymatic in function and that serve as a scaffold for various protein-protein interactions. This process aids in connecting the proximal to distal signaling pathways, thereby contributing to the full activation of T cells. This review provides a comprehensive snapshot of the various molecules involved in regulating T cell receptor signaling, covering both enzymes and adaptors, and will discuss their role in human disease.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

