JAK2V617F variant allele frequency >50% identifies patients with polycythemia vera at high risk for venous thrombosis
- PMID: 34897288
- PMCID: PMC8665926
- DOI: 10.1038/s41408-021-00581-6
JAK2V617F variant allele frequency >50% identifies patients with polycythemia vera at high risk for venous thrombosis
Abstract
Arterial (AT) and venous (VT) thrombotic events are the most common complications in patients with polycythemia vera (PV) and are the leading causes of morbidity and mortality. In this regard, the impact of JAK2V617F variant allele frequency (VAF) is still debated. The purpose of the current study was to analyze the impact of JAK2V617F VAF in the context of other established risk factors for thrombosis in a total of 865 2016 WHO-defined PV patients utilizing two independent cohorts: University of Florence (n = 576) as a training cohort and Policlinico Gemelli, Catholic University, Rome (n = 289) as a validation cohort. In the training cohort VT free-survival was significantly shorter in the presence of a JAK2V617F VAF > 50% (HR 4; p < 0.0001), whereas no difference was found for AT (HR 0.9; p = 0.8). Multivariable analysis identified JAK2V617F VAF > 50% (HR 3.8, p = 0.001) and previous VT (HR 2.2; p = 0.04) as independent risk factors for future VT whereas diabetes (HR 2.4; p = 0.02), hyperlipidemia (HR 2.3; p = 0.01) and previous AT (HR 2; p = 0.04) were independent risk factors for future AT. Similarly, JAK2V617F VAF > 50% (HR 2.4; p = 0.01) and previous VT (HR 2.8; p = 0.005) were confirmed as independent predictors of future VT in the validation cohort. Impact of JAK2V617F VAF > 50% on VT was particularly significant in conventional low-risk patients, both in Florence (HR 10.6, p = 0.005) and Rome cohort (HR 4; p = 0.02). In conclusion, we identified JAK2V617F VAF > 50% as an independent strong predictor of VT, supporting that AT and VT are different entities which might require distinct management.
© 2021. The Author(s).
Conflict of interest statement
PG received personal fees for advisory board and/or lectures from Novartis. VDS received personal fees for advisory board and/or lectures from AbbVie, AOP Orphan Pharmaceutical, and Novartis, and research grants from AbbVie and Novartis. AMV received personal fees for advisory board and/or lectures from Novartis, AbbVie, AOP Pharmaceuticals, BMS and Incyte. All other authors have no conflict to report.
Figures
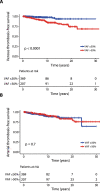

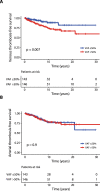
References
-
- Barbui T, Carobbio A, Rumi E, Finazzi G, Gisslinger H, Rodeghiero F et al. In contemporary patients with polycythemia vera, rates of thrombosis and risk factors delineate a new clinical epidemiology. Blood. 2014; 124. 10.1182/blood-2014-07-591610. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

