Inhibition of structural joint damage progression with upadacitinib in rheumatoid arthritis: 1-year outcomes from the SELECT phase 3 program
- PMID: 34897366
- PMCID: PMC9348768
- DOI: 10.1093/rheumatology/keab861
Inhibition of structural joint damage progression with upadacitinib in rheumatoid arthritis: 1-year outcomes from the SELECT phase 3 program
Abstract
Objectives: To evaluate the inhibition of progression of structural joint damage through week 48 in patients with moderately to severely active RA receiving upadacitinib as monotherapy or in combination with MTX.
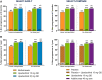
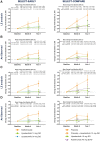
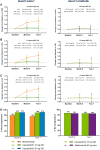
Methods: Radiographic progression was assessed in two phase 3 randomized controlled trials. MTX-naïve patients were randomized to upadacitinib 15 or 30 mg once daily or MTX monotherapy (SELECT-EARLY, n = 945), while MTX inadequate responders (IRs) were randomized to upadacitinib 15 mg once daily or adalimumab 40 mg every other week or placebo added to background MTX (SELECT-COMPARE, n = 1629). The mean changes from baseline in modified total Sharp score (mTSS), joint space narrowing and erosion scores were determined. Data were analysed both by linear extrapolation for missing data imputation and treatment switching and as observed.
Results: In patients naïve or with limited exposure to MTX (SELECT-EARLY), mean changes from baseline to week 48 in mTSS were 0.03 for upadacitinib 15 mg, 0.14 for upadacitinib 30 mg and 1.00 for MTX based on linear extrapolation (P < 0.001 for both upadacitinib doses vs MTX). Among patients with an inadequate response to MTX (SELECT-COMPARE), the mean change from baseline in mTSS was significantly reduced in the upadacitinib 15 mg plus MTX group vs placebo plus MTX (0.28 vs 1.73; P < 0.001). The mean change from baseline in the adalimumab plus MTX group was 0.39.
Conclusion: Upadacitinib monotherapy or in combination with background MTX was effective in inhibiting the progression of structural joint damage through week 48 in MTX-naïve and MTX-IR patients with RA.
Trial registration: ClinicalTrials.gov (https://clinicaltrials.gov), NCT02706873 and NCT02629159.
Keywords: DMARDs; biological therapies; outcome measures; radiology; rheumatoid arthritis.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures


References
-
- Wolfe F, Sharp JT.. Radiographic outcome of recent-onset rheumatoid arthritis: a 19-year study of radiographic progression. Arthritis Rheum 1998;41:1571–82. - PubMed
-
- Sokka T. Long-term outcomes of rheumatoid arthritis. Curr Opin Rheumatol 2009;21:284–90. - PubMed
-
- Smolen JS, Landewe RBM, Bijlsma JWJ. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. - PubMed
-
- Combe B, Lula S, Boone C, Durez P.. Effects of biologic disease-modifying anti-rheumatic drugs on the radiographic progression of rheumatoid arthritis: a systematic literature review. Clin Exp Rheumatol 2018;36:658–67. - PubMed
-
- Murray E, Ellis A, Butylkova Y. et al. Systematic review and network meta-analysis: effect of biologics on radiographic progression in rheumatoid arthritis. J Comp Eff Res 2018;7:959–74. - PubMed

