Immune cells in cardiac homeostasis and disease: emerging insights from novel technologies
- PMID: 34897403
- PMCID: PMC9020986
- DOI: 10.1093/eurheartj/ehab842
Immune cells in cardiac homeostasis and disease: emerging insights from novel technologies
Abstract
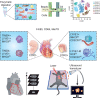
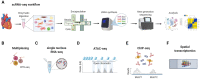
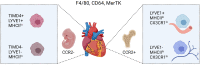
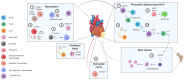
The increasing use of single-cell immune profiling and advanced microscopic imaging technologies has deepened our understanding of the cardiac immune system, confirming that the heart contains a broad repertoire of innate and adaptive immune cells. Leucocytes found in the healthy heart participate in essential functions to preserve cardiac homeostasis, not only by defending against pathogens but also by maintaining normal organ function. In pathophysiological conditions, cardiac inflammation is implicated in healing responses after ischaemic or non-ischaemic cardiac injury. The aim of this review is to provide a concise overview of novel methodological advancements to the non-expert readership and summarize novel findings on immune cell heterogeneity and functions in cardiac disease with a focus on myocardial infarction as a prototypic example. In addition, we will briefly discuss how biological sex modulate the cardiac immune response. Finally, we will highlight emerging concepts for novel therapeutic applications, such as targeting immunometabolism and nanomedicine.
Keywords: Bioimaging; Heart failure; Immunometabolism; Nanomedicine; Sexual dimorphism; Single-cell RNA sequencing.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2021. For permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Swirski FK, Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol 2018;18:733–744. - PubMed
-
- Jessup M, Brozena S. Heart failure. N Engl J Med 2003;348:2007–2018. - PubMed
-
- Lam CSP, Arnott C, Beale AL et al. Sex differences in heart failure. Eur Heart J 2019;40:3859c–3868c. - PubMed
-
- Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016;16:626–638. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

