Noninsect-Based Diet Leads to Structural and Functional Changes of Acidic Chitinase in Carnivora
- PMID: 34897517
- PMCID: PMC8789059
- DOI: 10.1093/molbev/msab331
Noninsect-Based Diet Leads to Structural and Functional Changes of Acidic Chitinase in Carnivora
Abstract
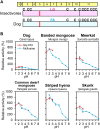
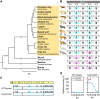
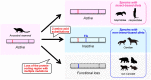
Acidic chitinase (Chia) digests the chitin of insects in the omnivorous stomach and the chitinase activity in carnivorous Chia is significantly lower than that of the omnivorous enzyme. However, mechanistic and evolutionary insights into the functional changes in Chia remain unclear. Here we show that a noninsect-based diet has caused structural and functional changes in Chia during the course of evolution in Carnivora. By creating mouse-dog chimeric Chia proteins and modifying the amino acid sequences, we revealed that F214L and A216G substitutions led to the dog enzyme activation. In 31 Carnivora, Chia was present as a pseudogene with stop codons in the open reading frame (ORF) region. Importantly, the Chia proteins of skunk, meerkat, mongoose, and hyena, which are insect-eating species, showed high chitinolytic activity. The cat Chia pseudogene product was still inactive even after ORF restoration. However, the enzyme was activated by matching the number and position of Cys residues to an active form and by introducing five meerkat Chia residues. Mutations affecting the Chia conformation and activity after pseudogenization have accumulated in the common ancestor of Felidae due to functional constraints. Evolutionary analysis indicates that Chia genes are under relaxed selective constraint in species with noninsect-based diets except for Canidae. These results suggest that there are two types of inactivating processes in Carnivora and that dietary changes affect the structure and activity of Chia.
Keywords: Chia; acidic chitinase; carnivores; digestive enzyme; gene loss; insectivores.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures



References
-
- Axelsson E, Ratnakumar A, Arendt ML, Maqbool K, Webster MT, Perloski M, Liberg O, Arnemo JM, Hedhammar A, Lindblad-Toh K.. 2013. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature 495(7441):360–364. - PubMed
-
- Boot RG, Blommaart EF, Swart E, Ghauharali-van der Vlugt K, Bijl N, Moe C, Place A, Aerts JM.. 2001. Identification of a novel acidic mammalian chitinase distinct from chitotriosidase. J Biol Chem. 276(9):6770–6778. - PubMed
-
- Boot RG, Bussink AP, Verhoek M, de Boer PA, Moorman AF, Aerts JM.. 2005. Marked differences in tissue-specific expression of chitinases in mouse and man. J Histochem Cytochem. 53(10):1283–1292. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

