Analysis of CDK12 alterations in a pan-cancer database
- PMID: 34898046
- PMCID: PMC8817093
- DOI: 10.1002/cam4.4483
Analysis of CDK12 alterations in a pan-cancer database
Abstract
Background: CDK12 inactivation leading to increased neoantigen burden has been hypothesized to sensitize tumors to immune checkpoint inhibition. Pan-cancer data regarding the frequency of CDK12 alterations are limited. We aimed to characterize CDK12 alterations across all cancer types through real-world clinical-grade sequencing.
Methods: This was a single-center retrospective analysis of 4994 cancer patients who underwent tissue or blood genomic profiling, including CDK12 assessment, conducted as part of routine care from December 2012 to January 2020. Prevalence, clinical characteristics, and treatment outcomes of patients with tumors with pathogenic CDK12 alterations were described.
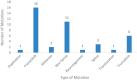
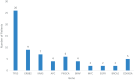
Results: In all, 39 (0.78%, n = 39/4994) patients had pathogenic CDK12 alterations. Among CDK12-altered tumors, the most common organ site was prostate (n = 9, 23.1%) followed by colorectal (n = 5, 12.8%). Adenocarcinoma was the most common histology (n = 26, 66.7%). Median follow-up from time of diagnosis was 4.02 years. Median overall survival from time of metastasis was 4.43 years (95% CI: 3.11-5.74). Ten patients with CDK12-altered tumors received at least one immune checkpoint inhibitor-containing regimen. The majority of patients (n = 6/10, 60%) experienced an objective response. Progression-free survival for patients who had metastatic disease and received a checkpoint inhibitor-containing regimen was 1.16 years (95% CI: 0.32-2.00).
Conclusion: CDK12 alterations are rare events across hematologic and solid tumor malignancies. They represent a clinically distinct molecular cancer subtype which may have increased responsiveness to checkpoint inhibition. Prospective studies are warranted to investigate checkpoint inhibition in CDK12-altered tumors.
Keywords: biomarkers; cancer genetics; clinical cancer research; genomics.
© 2021 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures