Characterization of a Triplet Vinylidene
- PMID: 34898204
- PMCID: PMC8704171
- DOI: 10.1021/jacs.1c11062
Characterization of a Triplet Vinylidene
Abstract
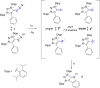
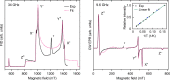
Singlet vinylidenes (R2C═C:) are proposed as intermediates in a series of organic reactions, and very few have been studied by matrix isolation or gas-phase spectroscopy. Triplet vinylidenes, however, featuring two unpaired electrons at a monosubstituted carbon atom are thus far only predicted as electronically excited-state species and represent an unexplored class of carbon-centered diradicals. We report the photochemical generation and low-temperature EPR/ENDOR characterization of the first ground-state high-spin (triplet) vinylidene. The zero-field splitting parameters (D = 0.377 cm-1 and |E|/D = 0.028) were determined, and the 13C hyperfine coupling tensor was obtained by 13C-ENDOR measurements. Most strikingly, the isotropic 13C hyperfine coupling constant (50 MHz) is far smaller than the characteristic values of triplet carbenes, demonstrating a unique electronic structure which is supported by quantum chemical calculations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Gomberg M. An instance of trivalent carbon: Triphenylmethyl. J. Am. Chem. Soc. 1900, 22, 757–771. 10.1021/ja02049a006. - DOI
-
- Dougherty D. A. Spin control in organic molecules. Acc. Chem. Res. 1991, 24, 88–94. 10.1021/ar00003a005. - DOI
-
- Bordon W. T., Ed.; Diradicals; Wiley-Interscience: New York, 1982.
-
- Igau A.; Grutzmacher H.; Baceiredo A.; Bertrand G. Analogous α,α’-bis-carbenoid, triply bonded species: synthesis of a stable λ3-phosphino carbene-λ5-phosphaacetylene. J. Am. Chem. Soc. 1988, 110, 6463–6466. 10.1021/ja00227a028. - DOI
LinkOut - more resources
Full Text Sources

