Distinct Interaction Mechanism of RNA Polymerase and ResD at Proximal and Distal Subsites for Transcription Activation of Nitrite Reductase in Bacillus subtilis
- PMID: 34898263
- PMCID: PMC8846475
- DOI: 10.1128/JB.00432-21
Distinct Interaction Mechanism of RNA Polymerase and ResD at Proximal and Distal Subsites for Transcription Activation of Nitrite Reductase in Bacillus subtilis
Abstract
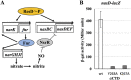
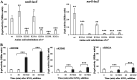
The ResD-ResE signal transduction system plays a pivotal role in anaerobic nitrate respiration in Bacillus subtilis. The nasD operon encoding nitrite reductase is essential for nitrate respiration and is tightly controlled by the ResD response regulator. To understand the mechanism of ResD-dependent transcription activation of the nasD operon, we explored ResD-RNA polymerase (RNAP), ResD-DNA, and RNAP-DNA interactions required for nasD transcription. Full transcriptional activation requires the upstream promoter region where five molecules of ResD bind. The distal ResD-binding subsite at -87 to -84 partially overlaps a sequence similar to the consensus distal subsite of the upstream (UP) element with which the Escherichia coli C-terminal domain of the α subunit (αCTD) of RNAP interacts to stimulate transcription. We propose that interaction between αCTD and ResD at the promoter-distal site is essential for stimulating nasD transcription. Although nasD has an extended -10 promoter, it lacks a reasonable -35 element. Genetic analysis and structural simulations predicted that the absence of the -35 element might be compensated by interactions between σA and αCTD and between αCTD and ResD at the promoter-proximal ResD-binding subsite. Thus, our work suggested that ResD participates in nasD transcription activation by binding to two αCTD subunits at the proximal and distal promoter sites, representing a unique configuration for transcription activation. IMPORTANCE A significant number of ResD-controlled genes have been identified, and transcription regulatory pathways in which ResD participates have emerged. Nevertheless, the mechanism of how ResD activates transcription of different genes in a nucleotide sequence-specific manner has been less explored. This study suggested that among the five ResD-binding subsites in the promoter of the nasD operon, the promoter-proximal and -distal ResD-binding subsites play important roles in nasD activation by adapting different modes of protein-protein and protein-DNA interactions. The finding of a new type of protein-promoter architecture provides insight into the understanding of transcription activation mechanisms controlled by transcription factors, including the ubiquitous response regulators of two-component regulatory systems, particularly in Gram-positive bacteria.
Keywords: Bacillus subtilis; RNA polymerase subunits; ResD-dependent transcription; UP element; nitrite reductase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


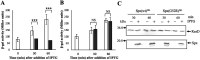


References
-
- Sun G, Birkey SM, Hulett FM. 1996. Three two-component signal-transduction systems interact for Pho regulation in Bacillus subtilis. Mol Microbiol 19:942–948. - PubMed
-
- Nakano MM, Zuber P. 2002. Anaerobiosis, p 393–404. In Sonenshein AL, Hoch JA, Losick R (ed), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

