Nonaqueous Solvent Extraction for Enhanced Metal Separations: Concept, Systems, and Mechanisms
- PMID: 34898845
- PMCID: PMC8662634
- DOI: 10.1021/acs.iecr.1c02287
Nonaqueous Solvent Extraction for Enhanced Metal Separations: Concept, Systems, and Mechanisms
Abstract
Efficient and sustainable separation of metals is gaining increasing attention, because of the essential roles of many metals in sustainable technologies for a climate-neutral society, such as rare earths in permanent magnets and cobalt, nickel, and manganese in the cathode materials of lithium-ion batteries. The separation and purification of metals by conventional solvent extraction (SX) systems, which consist of an organic phase and an aqueous phase, has limitations. By replacing the aqueous phase with other polar solvents, either polar molecular organic solvents or ionic solvents, nonaqueous solvent extraction (NASX) largely expands the scope of SX, since differences in solvation of metal ions lead to different distribution behaviors. This Review emphasizes enhanced metal extraction and remarkable metal separations observed in NASX systems and discusses the effects of polar solvents on the extraction mechanisms according to the type of polar solvents and the type of extractants. Furthermore, the considerable effects of the addition of water and complexing agents on metal separations in terms of metal ion solvation and speciation are highlighted. Efforts to integrate NASX into metallurgical flowsheets and to develop closed-loop solvometallurgical processes are also discussed. This Review aims to construct a framework of NASX on which many more studies on this topic, both fundamental and applied, can be built.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


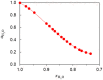
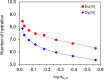
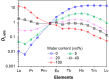


References
-
- Demopoulos G. P. Solvent Extraction in Precious Metals Refining. JOM 1986, 38 (6), 13–17. 10.1007/BF03257809. - DOI
-
- Saguru C.; Ndlovu S.; Moropeng D. A Review of Recent Studies into Hydrometallurgical Methods for Recovering PGMs from Used Catalytic Converters. Hydrometallurgy 2018, 182, 44–56. 10.1016/j.hydromet.2018.10.012. - DOI
-
- Nguyen V. T.; Riaño S.; Binnemans K. Separation of Precious Metals by Split-Anion Extraction Using Water-Saturated Ionic Liquids. Green Chem. 2020, 22 (23), 8375–8388. 10.1039/D0GC02356F. - DOI
-
- Cheng C. Y.; Barnard K. R.; Zhang W.; Robinson D. J. Synergistic Solvent Extraction of Nickel and Cobalt: A Review of Recent Developments. Solvent Extr. Ion Exch. 2011, 29 (5–6), 719–754. 10.1080/07366299.2011.595636. - DOI
-
- Banza A. N.; Gock E.; Kongolo K. Base Metals Recovery from Copper Smelter Slag by Oxidising Leaching and Solvent Extraction. Hydrometallurgy 2002, 67 (1), 63–69. 10.1016/S0304-386X(02)00138-X. - DOI
Publication types
LinkOut - more resources
Full Text Sources
