Transcutaneous Auricular Vagus Nerve Stimulation (tAVNS) Delivered During Upper Limb Interactive Robotic Training Demonstrates Novel Antagonist Control for Reaching Movements Following Stroke
- PMID: 34899170
- PMCID: PMC8655845
- DOI: 10.3389/fnins.2021.767302
Transcutaneous Auricular Vagus Nerve Stimulation (tAVNS) Delivered During Upper Limb Interactive Robotic Training Demonstrates Novel Antagonist Control for Reaching Movements Following Stroke
Abstract
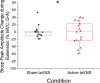
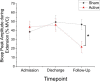
Implanted vagus nerve stimulation (VNS) delivered concurrently with upper limb rehabilitation has been shown to improve arm function after stroke. Transcutaneous auricular VNS (taVNS) offers a non-invasive alternative to implanted VNS and may provide similar therapeutic benefit. There is much discussion about the optimal approach for combining VNS and physical therapy, as such we sought to determine whether taVNS administered during robotic training, specifically delivered during the premotor planning stage for arm extension movements, would confer additional motor improvement in patients with chronic stroke. Thirty-six patients with chronic, moderate-severe upper limb hemiparesis (>6 months; mean Upper Extremity Fugl-Meyer score = 25 ± 2, range 13-48), were randomized to receive 9 sessions (1 h in length, 3x/week for 3 weeks) of active (N = 18) or sham (N = 18) taVNS (500 ms bursts, frequency 30 Hz, pulse width 0.3 ms, max intensity 5 mA, ∼250 stimulated movements per session) delivered during robotic training. taVNS was triggered by the onset of a visual cue prior to center-out arm extension movements. Clinical assessments and surface electromyography (sEMG) measures of the biceps and triceps brachii were collected during separate test sessions. Significant motor improvements were measured for both the active and sham taVNS groups, and these improvements were robust at 3 month follow-up. Compared to the sham group, the active taVNS group showed a significant reduction in spasticity of the wrist and hand at discharge (Modified Tardieu Scale; taVNS = -8.94% vs. sham = + 2.97%, p < 0.05). The EMG results also demonstrated significantly increased variance for the bicep peak sEMG amplitude during extension for the active taVNS group compared to the sham group at discharge (active = 26.29% MVC ± 3.89, sham = 10.63% MVC ± 3.10, mean absolute change admission to discharge, p < 0.01), and at 3-month follow-up, the bicep peak sEMG amplitude was significantly reduced in the active taVNS group (P < 0.05). Thus, robot training improved the motor capacity of both groups, and taVNS, decreased spasticity. taVNS administered during premotor planning of movement may play a role in improving coordinated activation of the agonist-antagonist upper arm muscle groups by mitigating spasticity and increasing motor control following stroke. Clinical Trial Registration: www.ClinicalTrials.gov, identifier (NCT03592745).
Keywords: hemiparesis; rehabilitation; robotic therapy; stroke; transcutaneous auricular vagus nerve stimulation (taVNS); vagus nerve stimulation (VNS).
Copyright © 2021 Chang, Coggins, Saul, Paget-Blanc, Straka, Wright, Datta-Chaudhuri, Zanos and Volpe.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Closed-loop transcutaneous auricular vagus nerve stimulation for the improvement of upper extremity motor function in stroke patients: a study protocol.Front Neurol. 2024 Jun 5;15:1379451. doi: 10.3389/fneur.2024.1379451. eCollection 2024. Front Neurol. 2024. PMID: 38903173 Free PMC article.
-
Motor Activated Auricular Vagus Nerve Stimulation as a Potential Neuromodulation Approach for Post-Stroke Motor Rehabilitation: A Pilot Study.Neurorehabil Neural Repair. 2023 Jun;37(6):374-383. doi: 10.1177/15459683231173357. Epub 2023 May 20. Neurorehabil Neural Repair. 2023. PMID: 37209010 Free PMC article. Clinical Trial.
-
Transcutaneous Auricular Vagus Nerve Stimulation with Concurrent Upper Limb Repetitive Task Practice for Poststroke Motor Recovery: A Pilot Study.J Stroke Cerebrovasc Dis. 2018 Jul;27(7):1998-2005. doi: 10.1016/j.jstrokecerebrovasdis.2018.02.056. Epub 2018 Mar 23. J Stroke Cerebrovasc Dis. 2018. PMID: 29580658 Clinical Trial.
-
Clinical Research Progress of the Post-Stroke Upper Limb Motor Function Improvement via Transcutaneous Auricular Vagus Nerve Stimulation.Neural Plast. 2023 Sep 25;2023:9532713. doi: 10.1155/2023/9532713. eCollection 2023. Neural Plast. 2023. PMID: 37789954 Free PMC article. Review.
-
Application of vagus nerve stimulation on the rehabilitation of upper limb dysfunction after stroke: a systematic review and meta-analysis.Front Neurol. 2023 Jun 21;14:1189034. doi: 10.3389/fneur.2023.1189034. eCollection 2023. Front Neurol. 2023. PMID: 37416314 Free PMC article.
Cited by
-
Neuroimmunomodulation of vagus nerve stimulation and the therapeutic implications.Front Aging Neurosci. 2023 Jul 6;15:1173987. doi: 10.3389/fnagi.2023.1173987. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37484689 Free PMC article. Review.
-
Transcutaneous auricular vagus nerve stimulation with task-oriented training improves upper extremity function in patients with subacute stroke: a randomized clinical trial.Front Neurosci. 2024 Mar 8;18:1346634. doi: 10.3389/fnins.2024.1346634. eCollection 2024. Front Neurosci. 2024. PMID: 38525376 Free PMC article.
-
Update on Non-invasive Brain Stimulation on Stroke Motor Impairment: A Narrative Review.Brain Neurorehabil. 2024 Jan 31;17(1):e5. doi: 10.12786/bn.2024.17.e5. eCollection 2024 Mar. Brain Neurorehabil. 2024. PMID: 38585032 Free PMC article. Review.
-
Two decades of vagus nerve stimulation for stroke: a bibliometric analysis.Front Neurol. 2025 Apr 4;16:1531127. doi: 10.3389/fneur.2025.1531127. eCollection 2025. Front Neurol. 2025. PMID: 40255891 Free PMC article.
-
Advances in Stroke Neurorehabilitation.J Clin Med. 2023 Oct 25;12(21):6734. doi: 10.3390/jcm12216734. J Clin Med. 2023. PMID: 37959200 Free PMC article. Review.
References
-
- American Heart Association [AHA] (2021). Heart Disease and Stroke Statistics- 2021 Heart Disease and Stroke Statistics Update Fact Sheet. Dallas: American Heart Association.
-
- Badran B. W., Dowdle L. T., Mithoefer O. J., LaBate N. T., Coatsworth J., Brown J. C., et al. (2018). Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: a concurrent taVNS/fMRI study and review. Brain Stimul. 11 492–500. 10.1016/j.brs.2017.12.009 - DOI - PMC - PubMed
-
- Burke D. (1988). Spasticity as an adaptation to pyramidal tract injury. Adv. Neurol. 47 401–423. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

