When Are Depolarizing GABAergic Responses Excitatory?
- PMID: 34899178
- PMCID: PMC8651619
- DOI: 10.3389/fnmol.2021.747835
When Are Depolarizing GABAergic Responses Excitatory?
Abstract
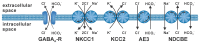
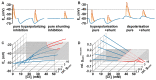
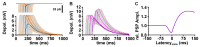
The membrane responses upon activation of GABA(A) receptors critically depend on the intracellular Cl- concentration ([Cl-]i), which is maintained by a set of transmembrane transporters for Cl-. During neuronal development, but also under several pathophysiological conditions, the prevailing expression of the Cl- loader NKCC1 and the low expression of the Cl- extruder KCC2 causes elevated [Cl-]i, which result in depolarizing GABAergic membrane responses. However, depolarizing GABAergic responses are not necessarily excitatory, as GABA(A) receptors also reduces the input resistance of neurons and thereby shunt excitatory inputs. To summarize our knowledge on the effect of depolarizing GABA responses on neuronal excitability, this review discusses theoretical considerations and experimental studies illustrating the relation between GABA conductances, GABA reversal potential and neuronal excitability. In addition, evidences for the complex spatiotemporal interaction between depolarizing GABAergic and glutamatergic inputs are described. Moreover, mechanisms that influence [Cl-]i beyond the expression of Cl- transporters are presented. And finally, several in vitro and in vivo studies that directly investigated whether GABA mediates excitation or inhibition during early developmental stages are summarized. In summary, these theoretical considerations and experimental evidences suggest that GABA can act as inhibitory neurotransmitter even under conditions that maintain substantial depolarizing membrane responses.
Keywords: KCC2; NKCC1; SLC12A2; SLC12A5; chloride homeostasis; gaba receptor; neuronal development.
Copyright © 2021 Kilb.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
GABAA-Receptor Signaling and Ionic Plasticity in the Generation and Spread of Seizures.In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 6. In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 6. PMID: 39637123 Free Books & Documents. Review.
-
Anomalous levels of Cl- transporters cause a decrease of GABAergic inhibition in human peritumoral epileptic cortex.Epilepsia. 2011 Sep;52(9):1635-44. doi: 10.1111/j.1528-1167.2011.03111.x. Epub 2011 Jun 2. Epilepsia. 2011. PMID: 21635237
-
Cl- uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1.J Physiol. 2004 Jun 15;557(Pt 3):829-41. doi: 10.1113/jphysiol.2004.062471. Epub 2004 Apr 16. J Physiol. 2004. PMID: 15090604 Free PMC article.
-
Hyperpolarizing GABAergic transmission depends on KCC2 function and membrane potential.Channels (Austin). 2011 Nov-Dec;5(6):475-81. doi: 10.4161/chan.5.6.17952. Epub 2011 Nov 1. Channels (Austin). 2011. PMID: 22082832 Free PMC article.
-
Development and regulation of chloride homeostasis in the central nervous system.Front Cell Neurosci. 2015 Sep 24;9:371. doi: 10.3389/fncel.2015.00371. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26441542 Free PMC article. Review.
Cited by
-
Pharmacological Analysis of GABAA Receptor and Sigma1R Chaperone Interaction: Research Report I-Investigation of the Anxiolytic, Anticonvulsant and Hypnotic Effects of Allosteric GABAA Receptors' Ligands.Int J Mol Sci. 2023 May 31;24(11):9580. doi: 10.3390/ijms24119580. Int J Mol Sci. 2023. PMID: 37298532 Free PMC article.
-
Editorial: Cellular and molecular mechanisms that govern assembly, plasticity, and function of GABAergic inhibitory circuits in the mammalian brain.Front Cell Neurosci. 2025 Feb 11;19:1568845. doi: 10.3389/fncel.2025.1568845. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40007758 Free PMC article. No abstract available.
-
GABAergic integration of transient and persistent neurons in the developing mouse somatosensory cortex.Front Cell Neurosci. 2025 Feb 26;19:1556174. doi: 10.3389/fncel.2025.1556174. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40078325 Free PMC article.
-
Loss of KCC2 in GABAergic Neurons Causes Seizures and an Imbalance of Cortical Interneurons.Front Mol Neurosci. 2022 Mar 16;15:826427. doi: 10.3389/fnmol.2022.826427. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35370549 Free PMC article.
-
Delaying the GABA Shift Indirectly Affects Membrane Properties in the Developing Hippocampus.J Neurosci. 2023 Jul 26;43(30):5483-5500. doi: 10.1523/JNEUROSCI.0251-23.2023. Epub 2023 Jul 12. J Neurosci. 2023. PMID: 37438107 Free PMC article.
References
-
- Aronica E., Boer K., Redeker S., Spliet W. G. M., van Rijen P. C., Troost D., et al. . (2007). Differential expression patterns of chloride transporters, Na+-K+-2Cl−-cotransporter and K+-Cl−-cotransporter, in epilepsy-associated malformations of cortical development. Neuroscience 145, 185–196. 10.1016/j.neuroscience.2006.11.041 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

