Dynamic Diversity of Glial Response Among Species in Spinal Cord Injury
- PMID: 34899275
- PMCID: PMC8662749
- DOI: 10.3389/fnagi.2021.769548
Dynamic Diversity of Glial Response Among Species in Spinal Cord Injury
Abstract
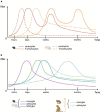
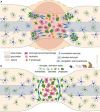
The glial scar that forms after traumatic spinal cord injury (SCI) is mostly composed of microglia, NG2 glia, and astrocytes and plays dual roles in pathophysiological processes induced by the injury. On one hand, the glial scar acts as a chemical and physical obstacle to spontaneous axonal regeneration, thus preventing functional recovery, and, on the other hand, it partly limits lesion extension. The complex activation pattern of glial cells is associated with cellular and molecular crosstalk and interactions with immune cells. Interestingly, response to SCI is diverse among species: from amphibians and fishes that display rather limited (if any) glial scarring to mammals that exhibit a well-identifiable scar. Additionally, kinetics of glial activation varies among species. In rodents, microglia become activated before astrocytes, and both glial cell populations undergo activation processes reflected amongst others by proliferation and migration toward the injury site. In primates, glial cell activation is delayed as compared to rodents. Here, we compare the spatial and temporal diversity of the glial response, following SCI amongst species. A better understanding of mechanisms underlying glial activation and scar formation is a prerequisite to develop timely glial cell-specific therapeutic strategies that aim to increase functional recovery.
Keywords: glial bridge; glial cells; glial scar; immune cells; primates; regenerative species; rodents; spinal cord injury (SCI).
Copyright © 2021 Perez, Gerber and Perrin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Beck K. D., Nguyen H. X., Galvan M. D., Salazar D. L., Woodruff T. M., Anderson A. J. (2010). Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 133 433–447. 10.1093/brain/awp322 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

